Chemical Evolution of Groundwater in the Dindefello Plain Area in South-Eastern Senegal ()
1. Introduction
The composition of groundwater is an important source of hydrogeochemical information about earth surface weathering processes [1] . Hence, knowledge of the solute constituents could aid in reconstructing the major chemical reactions contributing to groundwater mineralization ( [2] - [8] ). The purpose of the exercise was to demonstrate a proposed hypothesis of Diop et al. [9] , that the main control on groundwater mineralization in the near surface aquifers of the DPA hydrologic system (hereinafter referred to as DPA-HS) is the pH-driven process of mineral breakdown (mainly carbonates and silicates), under the carbon dioxide regime. This paper is based on the interpretation of water composition data presented by these authors, and it follows a two-step procedure.
In the first instance, key diagrams pertinent for inferring the hydrogeochemical evolution of groundwater in the DPA-HS are presented, and then the batch modeling procedure of PHREEQC computer program (version 2, [10] ) to reconstruct the hypothesized reaction models is run. In essence, this simulated the specific case that recharge spring water infiltrating into the ground interacts along its flow paths with calcite, dolomite and albite minerals under “open-system conditions” [4] to account for the observed chemical composition of groundwater downstream.
2. Location and Hydrogeology
The study area is located on the Senegal-Guinea Republic border, between longitude 12˚20'W - 12˚25'W and latitude 12˚20'N - 12˚25'N, within a region referred to here as the “Dindefello Plain Area”―DPA.
The DPA is a poorly documented depression bounded to the south by a range of hills rising to over 400 m above the surrounding terrain, with maximum elevations approximately 450 m above MSL (Figure 1). Fracture and contact springs are found at the margins of the top of the plateau and its foothills. The regional structures are components of the Fouta Djalon Mountains chain (Guinea Republic), which is emplaced on the southern prolongation of the Mauritanides fold belt ([11] [12] ). Organically, the Mauritanides belt flanks the 2 × 109
![]()
Figure 1. Location map of the study area within south-eastern Senegal.
years old West African craton to the west ( [13] [14] ). Rocks underlying the DPA, or outcropping, are mainly young basement rocks of the Ségou-group ([15] -[18] ) which contain mineralogical groupings (carbonaceous limestones and dolostones and feldspatic sandstones assemblages) that are source of major ions in the groundwater of the study area.
The groundwater system underlying the DPA comprises the low-permeability regolith zone (upper aquifer unit with the water table mostly 10 - 30 m below ground surface), and the more productive adjacent zone reservoir of bedrock disintegration and leaching (lower aquifer unit). There is interaction between this system and the topography-driven spring water system that flows out of the uplands and avalanches down into the plain area.
Recharge to the subterranean drainage system occurs on a yearly basis, as spring discharges cascade down onto the valley floors and drain into pathways incised by the river system, and remain in the channel over a long distance. However, the low permeability character of the superficial regolith reduces the effectiveness of direct rainwater infiltration. Thus, groundwater is recharged locally and essentially by runoff in the alluvium of rivers and their tributaries, which have eroded their ways into the plain floor. Spring water percolating downward through the alluvium of river channels reaches the groundwater table, thereby forming a major source of groundwater recharge. The hydrodynamic regime of the hydrologic system is topography-driven and the land surface can be used to infer groundwater flow patterns, assuming that the water table is a subdued replica of the surface topography.
In the study area domestic water supply is typically obtained from shallow hand-dug wells bored in the regolith zone reservoir, down to depths of 20 - 30 m below ground surface. The water level in these wells can rise 10 m during the rainy season and then can fall almost rapidly. However, a more permanent supply is reached by motor-driven wells that penetrate the deeper seated fractured zone reservoir. During the rainy season from May to October, this region receives about 1200 mm rainfall, of which nearly 20% may result as net rain [19] . During the dry season, surface water sources are very limited, and the depth of groundwater is relatively great (often >25 - 30 m). To meet their water needs in this period, people most frequently collect water from springs and pondlets dug on stream beds.
3. Materials and Methods
Traditionally, the geochemistry of groundwater can be used to reconstruct the controlling processes of mineral dissolution. Theories on the origin of groundwater mineralization generally agree that when water comes into contact with minerals, chemical weathering and dissolution of the minerals begins and continues until equilibrium concentration is attained in the water, or until all minerals are consumed [4] . Thus, depending on the environment, chemical and mineralogical characteristics of the rocks which groundwater has come into contact with during its flow history, its chemistry may be altered to reflect the host rocks. Because of this, one inclination might be to use the equilibrium concept as a means of interpreting chemical data. So, if water samples need to be to checked as to how far the hypothetical reactions have proceeded to give their hydrochemistry, their SIs with respect to these minerals should be calculated. For a given water sample, its SI with respect to any candidate source or sink mineral can be computed, based on the ionic product for the aqueous phase and its equilibrium constant at the considered temperature.
It is not intended here to describe the theoretical background (for more details, see [4] , [20] or [21] ), but, instead, to note that the saturation state for a particular mineral (considered as possible source or sink of ions to aqueous solution), or other participating substance is expressed as saturation index (SI), [20] :
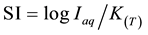
where:
Iap: ionic activity product
KT: mineral equilibrium constant at the specified temperature.
• For SI > +0.1, the specific mineral is supersaturated, and precipitation is possible;
• For −0.1 < SI < +0.1, the specific mineral is saturated and precipitation or dissolution possible; and
• For SI < −0.1, the specific mineral is undersaturated, and dissolution possible.
The approach taken was to first carry out exploratory investigations for mineral-water relationships from the analyzed data so as to create work assumptions, and then use the computer program PHREEQC (http://water.usgs.gov/software/) to check these assumptions.
The data used for this study (Table 1) were essentially the major elements’ chemistry of water samples from springs, water supply pondlets dug by villagers on stream beds and village hand-dug wells. Figure 2 conveys the areal distribution of the various samples. Supplementary data included the chemical composition of rainfall [22] which was found to be available and so allowed comparison with spring water (Table 1), as well as compiling qualitative data about the nature of the physical environment. This enabled approximating groundwater flow patterns to be drawn (by assuming that the water table is controlled by the surface topography), thus providing a comprehensive inventory of potential reactants for the proposed geochemical model evolution.
4. Results and Discussions
4.1. Analysis of Trend
The data employed in this study is as presented in Table 1. The waters have acidity at near neutral pH (4.65 ≤ pH ≤ 6.93), and total mineralization (TDS) ranged from 20 to 570 mg/L. The five elements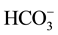 , SiO2, Ca2+, Mg2+ and Na+, in order of decreasing abundance, make up the bulk of mineralization. These elements represent over 80% - 90% of the TDS. In a general way, subsurface groundwater (i.e. water samples from hand-dug wells) has much higher mineralization than spring water, which shows near-local rainwater characteristics. The consistently lower mineralization of spring water indicated rapid drainage of meteoric waters through the high permeability pathways provided by rock fractures, joints, bedding planes and faults in the highlands. This rapid flow results in shorter residence time and less mineral-water interaction in the host rocks. However, anomalously high TDS of some springs (Pr3 and 14) and pondlets (Pr4 and 5) revealed the influence of marshy conditions prevailing at these locations where water may be acidic from CO2 production (from organic matter decay) leading to increased solubility. In contrast, groundwater travelling in the plain aquifers generally had increasing levels of dissolved solids with distance down gradient as a result of chemically evolved recharge waters from longer residence time. Consequently, a model of hydrochemical evolution must explain the increase in dissolved solids, and hence the evolution of recharge spring water (or meteoric water), as it avalanches down onto the valley floor and infiltrates into the ground to become subsurface groundwater.
, SiO2, Ca2+, Mg2+ and Na+, in order of decreasing abundance, make up the bulk of mineralization. These elements represent over 80% - 90% of the TDS. In a general way, subsurface groundwater (i.e. water samples from hand-dug wells) has much higher mineralization than spring water, which shows near-local rainwater characteristics. The consistently lower mineralization of spring water indicated rapid drainage of meteoric waters through the high permeability pathways provided by rock fractures, joints, bedding planes and faults in the highlands. This rapid flow results in shorter residence time and less mineral-water interaction in the host rocks. However, anomalously high TDS of some springs (Pr3 and 14) and pondlets (Pr4 and 5) revealed the influence of marshy conditions prevailing at these locations where water may be acidic from CO2 production (from organic matter decay) leading to increased solubility. In contrast, groundwater travelling in the plain aquifers generally had increasing levels of dissolved solids with distance down gradient as a result of chemically evolved recharge waters from longer residence time. Consequently, a model of hydrochemical evolution must explain the increase in dissolved solids, and hence the evolution of recharge spring water (or meteoric water), as it avalanches down onto the valley floor and infiltrates into the ground to become subsurface groundwater.
Moreover, Table 1 shows that the higher its mineralization grade (column 3), the more groundwater is approaching equilibrium/saturation with respect to calcite, dolomite and aqueous silica (column 13 to 20). It seems reasonable, therefore, to suggest these three minerals as possible sources of dissolved constituents for groundwater. Figure 2 and Figure 3 represent key diagrams as regards the geochemical conditions giving rise to groundwater chemistry.
Figure 3 indicates the major compositional water types observed by plotting the samples in a Piper diagram whereby the circle radius is a scale for TDS. The chemical composition of most spring samples shows a minor nitrate and chloride impairment (possibly due to human impact), that causes them to vaguely shift along the (SO4 + Cl + NO3)-axis of the lozenge and slightly obfuscate the evolutionary path for cations. The grouping shows:
1) Waters whose solutes are dominated by ; These are waters in which calcium and magnesium amount 50% or more of the cation budget, and bicarbonate to more than 50% of the anions. They are typical of recharge water into carbonate hosted lithology, viz. a geological environment containing calcite and dolomite whereby the characteristically high
; These are waters in which calcium and magnesium amount 50% or more of the cation budget, and bicarbonate to more than 50% of the anions. They are typical of recharge water into carbonate hosted lithology, viz. a geological environment containing calcite and dolomite whereby the characteristically high 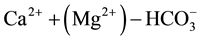 levels suggest the geochemical implication of gas
levels suggest the geochemical implication of gas 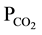 as a controlling factor.
as a controlling factor.
2) Waters that are relatively enriched in alkali ions, comprising two cases of sodium bicarbonate end-member type, ; Theses are waters in which milliequivalents of [Na+ + (K+)] and
; Theses are waters in which milliequivalents of [Na+ + (K+)] and 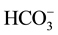 contribute each more than 50% of the ion budget. They are distributed along the (Na + K)-axis of the Piper diagram in Figure 3, a distribution pattern that suggests chemically evolved type (1) waters through contemporaneous interaction with silicate rocks.
contribute each more than 50% of the ion budget. They are distributed along the (Na + K)-axis of the Piper diagram in Figure 3, a distribution pattern that suggests chemically evolved type (1) waters through contemporaneous interaction with silicate rocks.
Figure 4 shows in this respect that the water composition, expressed in terms of SiO2 and Na+/H+, plots towards the boundary between kaolinite-Na-monmorillonite for spring waters, as compared to groundwater that characteristically evolves to the albite boundary as mineralization proceeds. Presumably albite dissolves incongruently to produce dissolved SiO2 and Na in spring waters in the early stage of mineralization, and both kaolinite and Na-montmorillonite as secondary minerals by-products. The kaolinitic and Na-montmorillonitic by- products may dissolve subsequently as groundwater mineralization progresses. Several authors ([1] [3] [7] ) have observed this behavior pattern in igneous terrains where most groundwater-derived surface waters, such as
![]()
Figure 2. Location of the sampling sites within the DPA physiographic region. Note: The arrow indicates the simulated reaction flow path. Refer to Figure 1 for the location of Pr13 (Tiangué Village).
![]()
Figure 3. Piper diagram for the various water samples from the DPA- HS, with TDS as circles. Note: Triangles are springs water samples and circles represent groundwater samples.
![]()
Figure 4. Stability diagram for gibbsite, kaolinite, montmorillonite and albite at 25˚C. Note: Triangles are samples from springs, and circles re- present samples from groundwater.
springs and baseflow, plot in the kaolinite fields of stability of this diagram type.
In Figure 5 the water chemistry expressed in terms of pH and 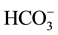 is compared to the simple open-sys- tem dissolution model of [4] describing the evolution pattern of groundwater in contact with calcite at 15˚C and under various partial pressures. It is obvious in this figure that all water samples plot above the atmospheric CO2 partial pressure (
is compared to the simple open-sys- tem dissolution model of [4] describing the evolution pattern of groundwater in contact with calcite at 15˚C and under various partial pressures. It is obvious in this figure that all water samples plot above the atmospheric CO2 partial pressure ( ) level of 10−3.5 bar; viz. within
) level of 10−3.5 bar; viz. within 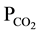 levels in ranges 10−1 - 10−3 bar which are at least 30-times the atmospheric level. This clearly means that water infiltrating through the aquifer soil zones become charged with CO2 gas that normally makes water acidic and prone to weathering. Indeed, CO2 normally reacts with water to form carbonic acid (H2CO3 ), which initiates geochemical reactions in groundwater.
levels in ranges 10−1 - 10−3 bar which are at least 30-times the atmospheric level. This clearly means that water infiltrating through the aquifer soil zones become charged with CO2 gas that normally makes water acidic and prone to weathering. Indeed, CO2 normally reacts with water to form carbonic acid (H2CO3 ), which initiates geochemical reactions in groundwater.
It is obvious in Figure 5 that spring waters occur with the lowest values of pH and , while groundwater samples plot on a position near the calcite saturation line, thereby evolving within
, while groundwater samples plot on a position near the calcite saturation line, thereby evolving within ![]() paths lines in the range roughly from 10−1 to 10−2 bar. Furthermore, the comparison in Figure 5 also shows a regular increase in
paths lines in the range roughly from 10−1 to 10−2 bar. Furthermore, the comparison in Figure 5 also shows a regular increase in ![]() with increasing pH (in the range 6 - 7) of groundwater where the higher its CO2 partial pressure, the more groundwater is nearing equilibrium with respect to calcite. It is very likely that the hydrologic field situation for the dissolution of calcite to proceed roughly follows the evolution pattern described by the arrow. This trend has been interpreted to mean the conventional view point that more calcite dissolution leads to higher H+ consumption and, therefore, to higher pH and
with increasing pH (in the range 6 - 7) of groundwater where the higher its CO2 partial pressure, the more groundwater is nearing equilibrium with respect to calcite. It is very likely that the hydrologic field situation for the dissolution of calcite to proceed roughly follows the evolution pattern described by the arrow. This trend has been interpreted to mean the conventional view point that more calcite dissolution leads to higher H+ consumption and, therefore, to higher pH and ![]() values, all according to the reaction:
values, all according to the reaction: ![]() .
.
As the dissolution reaction for calcite proceeds, consumption of H+ (equivalent to a pH rise) normally causes the concentration of H2CO3 to decline however (according to: ![]() ) by fairly uniform CO2 partial pressure within the range 10−1 - 10−2 bar. This implies that replenishment of the CO2 stock (a CO2 refill process from outside the system) must occur simultaneous as dissolved CO2 is destroyed progressively on dissolution of calcite. Presumably, spring water regularly seeping into the ground as recharge water is an important source of CO2 for groundwater. However, reaction types that tend to prevent a partial pressure drop within the system may include the conversion of calcite to wollastonite:
) by fairly uniform CO2 partial pressure within the range 10−1 - 10−2 bar. This implies that replenishment of the CO2 stock (a CO2 refill process from outside the system) must occur simultaneous as dissolved CO2 is destroyed progressively on dissolution of calcite. Presumably, spring water regularly seeping into the ground as recharge water is an important source of CO2 for groundwater. However, reaction types that tend to prevent a partial pressure drop within the system may include the conversion of calcite to wollastonite: ![]() [23] and this factor is not included in this discussion. It is worth noting from the development above that
[23] and this factor is not included in this discussion. It is worth noting from the development above that ![]() is from two different sources: 1) ionization of H2CO3 (
is from two different sources: 1) ionization of H2CO3 (![]() ), and 2) reaction of H+ with calcite (
), and 2) reaction of H+ with calcite (![]() ), which probably explains its predominance in groundwater.
), which probably explains its predominance in groundwater.
Additional sources of solute constituents in groundwater may include the weathering of other silicate compounds known to be present in the soil zone (e.g., potassium feldspar-KAlSi3O8) which is much less reactive than sodium feldspar―NaAlSi3O8 [2] . Also, aluminosilicate/clays minerals, as well as the dissolution of gypsum and cationic exchange, could be sources of solute in groundwater. Representing these reactions ( [4] [5] ) as in Table 2 supports the contention that these are, perhaps, the more likely reactions to consider in explaining the major-ion chemical evolution of groundwater in the DPA. These reactions fairly illustrate how H+ ions might be
![]()
Figure 5. Plot of ![]() as a function of pH according to the open-system dissolution model of Freeze and Cherry [4] . Note: Triangles are springs water samples and rectangles represent groundwater samples: The waters approach calcite saturation as dissolved
as a function of pH according to the open-system dissolution model of Freeze and Cherry [4] . Note: Triangles are springs water samples and rectangles represent groundwater samples: The waters approach calcite saturation as dissolved ![]() and H+ increase.
and H+ increase.
![]()
Table 2. Set of possible chemical reactions which may control the hydrochemistry in aquifers of the DPA.
produced and consumed upon dissolution of the hypothesized minerals to result in the observed major ions’ chemistry of groundwater.
4.2. Flow Path Modeling
The present research strongly suggests the CO2-driven process of mineral weathering as being the most likely mechanism in controlling the hydrochemical evolution in the DPA. This is in keeping with the established views ([23] [24] ) that CO2-charged recharge waters from rain percolating into subsurface soils naturally dissolve mineral materials during their underground movement. The mechanisms occur either by equilibrium with soil zone CO2 reservoir (or so-called “open system” conditions) or isolated from this reservoir (“closed system” conditions).
In the following assessment, the computer program PHREEQC (version 2; [10] ) was used to investigate the control of hypothesized reactions on the water chemistry. This involved modelling the specific case where spring waters that are chemically identical to rain waters infiltrating at foothills (around sampling site Pr1), interact along their flow paths with the said minerals (viz. calcite, dolomite, albite and gibbsite) under “open-system” conditions to produce the major ion composition of groundwater sampled from a hand-dug well at site Pr15 (Pelel village), at about 3.5 km downstream of Pr1; (refer to Figure 2 for the considered flow direction). This means that PHREEQC calculates speciation and moles of specified minerals and gas phases that would react with water sample Pr1 (initial solution) to produce an aqueous solution (final solution) whose mineral phases’ SIs with respect to the considered match those for water sample Pr15.
The steps for the model implementation (see Appendix) are: 1) Definition of the numerical entities for running the model; 2) Input of the required information; and 3) Execute the simulation.
Once the calculations have been fulfilled to target saturation constraints (as defined by the modelling procedure under the heading “EQUILIBRIUM PHASES 1”; see Box 1 of List 1 in the Appendix), PHREEQC displays the output results.
The output file is lengthy and contains a wealth of information that is not presented here for the sake of brevity. For more details, see List 1 (Appendix), where the focal outcome is given in Box 3, under the subheadings “Phase assemblage” and “Solution composition”. Moreover, the chemical analysis of Pr15 was also included in the model for comparison with results of PHREEQC speciation for the output solution (see Box 4.2 of List 1 in the Appendix). As can be viewed from the comparison therein, the model reproduces the observed water chemistry reasonable well.
Table 3 is a shortcut for this comparison. It should be noted that the ionic increase from sample Pr1 to sample Pr15 was modelled (see column 2 of the table) as a down-gradient chemical change in the aquifer due to a greater magnitude of water-mineral-interaction along flow path; essentially carbonate and silicate minerals. Clearly, the model results (column 2) are in good agreement with the chemistry of the groundwater collected at site Pr15 (column 3). An illustration of the good quality of the prediction is given in Figure 6 which shows the coefficient of correlation as 0.999, proving that the model reasonably reproduced the observed water chemistry.
Table 4 contains results from the mass balance. As can be seen, the amount of dissolved albite almost equals that of gibbsite that precipitates, indicating an inverse process. However, the (slight) discrepancy between the Na+ content generated by the model and the observed one (see also Table 3) suggests other sources of Na in solution, like cationic exchange process. In contrast to Na, predicted and observed concentration for both Ca and Mg match quite well.
Moreover, Table 4 provides an estimate of the contribution of dissolved CO2 to alkalinity (![]() ). The value so determined is 2.27 mmol/L (99.88 mg C/L as CO2), a plausible range of CO2 gas in the soil zone [20] . For the studied case, input CO2 gas provides nearly 42% of the total alkalinity source, which demonstrates its important role in the mineralization of groundwater in the considered system.
). The value so determined is 2.27 mmol/L (99.88 mg C/L as CO2), a plausible range of CO2 gas in the soil zone [20] . For the studied case, input CO2 gas provides nearly 42% of the total alkalinity source, which demonstrates its important role in the mineralization of groundwater in the considered system.
Overall, the stated mass balance would mean that the down-gradient well chemistry at sampling location Pr15 equals the up-gradient spring water chemistry at location Pr1 plus phases dissolving, minus phases precipitating. However, precipitation is negligible, as is the case for gibbsite (see List 1). For instance, the total alkalinity con- centration (405.46 mg C/L) given in Table 3 for sample Pr15 should be equal to the up-gradient carbon concentration of sample Pr1 (=14.13 mg C/L), plus the carbon from calcite and dolomite dissolution [3.21 mmole ![]() × 60 g/mole = 192.6 mg C/L] plus the carbon from dissolution of aqueous CO2 [2 × 2.27 mmole CO2 × 44 g/mole = 199.76 mg C/L] = 406.49 mg C/L. The two values agree well, as can be inferred from results of the mass balances calculations shown on Table 4.
× 60 g/mole = 192.6 mg C/L] plus the carbon from dissolution of aqueous CO2 [2 × 2.27 mmole CO2 × 44 g/mole = 199.76 mg C/L] = 406.49 mg C/L. The two values agree well, as can be inferred from results of the mass balances calculations shown on Table 4.
![]()
Table 3. Set of element concentrations essential for corroborating model results.
Note: The ionic increase from sample Pr1 to sample Pr15 was modeled (column 2) as evolutionary change in the aquifer due to a greater magnitude of water-mineral-interaction along flow path; essentially carbonate and silicate minerals.
![]()
Figure 6. Correlation between the observed and modeled six elements’ concentrations: Note: The fitted line is not statistically distinct from the line of one-to-one.
![]()
Table 4. Selected results from the mass balance calculations.
Note: Changes in saturation state D(SI) indicate possible chemical reaction of the specific mineral phase in the aquifers. A positive value implies mineral dissolution and a negative value indicates mineral precipitation.
5. Conclusions
In terms of the interpretation of the major ion loading of groundwater in the DPA and the geochemical reactions paths modelling, the conclusions of this study are as follows:
1) Chemical data analysis through graphical plots and aqueous speciation calculations revealed that the predominant groundwater reactions were the carbon dioxide-driven processes of carbonate and silicate minerals weathering that produced the calcium-magnesium-bicarbonate and sodium-bicarbonate waters which showed near saturation with respect to the minerals calcite and dolomite and albite among others; and
2) The PHREEQC batch modelling, which simulated the observed flow path data, supported the hypotheses on the stages of hydrogeochemical evolution of groundwater.
Acknowledgements
The work benefitted significantly from discussions with Prof. Dr. F. Wisotzky of the RU Bochum (RFG), who we wish to thank.
Appendix
In this appendix details are given of the model implementation and output result (List 1, below). More details on the computation procedure can be found in the tutorial [9] .
The heading “Reading data base” (Bloc 1) defines the algorithms here used for simulating the reactions path. The heading “Reading input data for simulation 1” (Bloc 2) introduces the essential data supplied to the model, namely: the initial solution (SOLUTION 1), representing hereinafter water sample Pr1 and the set of phases (“EQUILIBRIUM PHASES 1”) that are supposed to react during the batch reaction to turn the initial solution to a mineral saturation state matching that of water sample Pr15 (final solution). Thus, the keyword “SOLUTION 1” defines the composition of sample Pr1 and “EQUILIBRIUM PHASES 1” is the keyword that declares the list of phases used in the model and their respective SI. These so-called “target SIs values” were anticipated from a PHREEQC speciation of water sample Pr15. They are entered in the model to thermodynamically constraints the system. So conceptually, we consider the chemical balance that would result if water sample Pr1 were placed in a beaker which is regulated at 20˚C and allowed to react with the specified minerals and gas phases until the saturation limits are attained.
“Beginning of initial solution calculations” (Bloc 3) specifies the conversion of the input concentration data for Pr1 into molality (subheading “solution composition”). Subheading “Description of solution” introduces some basic properties calculated by the model. Subheading “Distribution of species” lists the different elements (master species) and amount of corresponding aqueous species in the solution. Subheading “Saturations indices” lists the SI calculations for all minerals that are appropriate for the aqueous solution and their respective formula.
The heading “Beginning of the batch reaction” (Bloc 4) defines the beginning of the batch reaction calculations for the simulation. When the simulation begins the initial solution and pure phases are put together and allowed to react until the saturation limits utilized to thermodynamically constraint the system (stated target SI values) are attained. The proportion of reactant is one mole for each mineral phase.
After the calculations are completed, the numbers of moles consumed for the reactions (mass transfers) are presented under subheading “Phase assemblage” (see Bloc 4.1; whereby positive delta value indicates precipitation and negative delta value dissolution). The resulting water chemistry (output or final solution) is then computed the (see subheading “Solution composition”; Bloc 4.2). Next, PRHEEQC calculates the resultant aqueous speciation (see subheading “Distribution of species”, Bloc 4.4) and then provides SIs for possible and impossible sources or sinks of solutes (see Subheading “Saturation indices”, Bloc 4.5).
List 1: Output data file for the modeled flow path
Input file: phreeqc.tmp
Output file: C:\Programme\Phreeqc\senegal1.out
Database file: C:\Programme\Phreeqc\Phreeqc.dat
------------------
Reading data base. (BLOC 1)
------------------
SOLUTION_MASTER_SPECIES
SOLUTION_SPECIES
PHASES
EXCHANGE_MASTER_SPECIES
EXCHANGE_SPECIES
SURFACE_MASTER_SPECIES
SURFACE_SPECIES
RATES
END
------------------------------------
Reading input data for simulation 1. (BLOC 2)
------------------------------------
TITLE Analyse Senegal Pr. 1 (BLOC 2.1)
SOLUTION 1
units mg/l
pH 5.89
pe 4.0
density 1.000
temp 20.0 # estimated
Ca2+ 1.92
Mg2+ 0.51
Na+ 0.28
K+ 0.43
Fe2+ 0.001
Mn2+ 0.001
Sr2+ 0.001
Cl- 0.98
C 14.13 as CO2 # from ionic balance
S6+ 1.27
N5+ 1.43 as NO3-
Si4+ 16.77 as SiO2
Al3+ 0.02
B3+ 0.22
EQUILIBRIUM_PHASES 1 (BLOC 2.2)
Calcite -0.53
Dolomite -1.58
Albite -1.86
Al(OH)3(a) -2.16
CO2(g) -1.04
END
-----
TITLE Analyse Senegal Diop Pr. 1
-----
-------------------------------------------
Beginning of initial solution calculations. (BLOC 3)
-------------------------------------------
Initial solution 1.
------------------Solution composition--------------(BLOC 3.1)----
Elements Molality Moles
Al3+ 7.413e-07 7.413e-07
B3+ 2.035e-05 2.035e-05
C4+ 3.211e-04 3.211e-04
Ca2+ 4.791e-05 4.791e-05
Cl- 2.764e-05 2.764e-05
Fe3+ 1.791e-08 1.791e-08
K+ 1.100e-05 1.100e-05
Mg2+ 2.098e-05 2.098e-05
Mn2+ 1.820e-08 1.820e-08
N5+ 2.306e-05 2.306e-05
Na+ 1.218e-05 1.218e-05
S6+ 1.322e-05 1.322e-05
Si4+ 2.791e-04 2.791e-04
Sr2+ 1.141e-08 1.141e-08
----------------------Description of solution------------- (BLOC 3.2)----
pH = 5.890
pe = 4.000
Activity of water = 1.000
Ionic strength = 2.415e-04
Mass of water (kg) = 1.000e+00
Total alkalinity (eq/kg) = 7.951e-05
Total CO2 (mol/kg) = 3.211e-04
Temperature (deg C) = 20.000
Electrical balance (eq) = 6.611e-06
Percent error, 100*(Cat-|An|)/(Cat+|An|) = 2.07
Iterations = 10
Total H = 1.110137e+02
Total O = 5.550824e+01
--------------------Distribution of species-------------- (BLOC 3.3)---
Log Log Log
Species Molality Activity Molality Activity Gamma
H+ 1.311e-06 1.288e-06 -5.882 -5.890 -0.008
OH- 5.364e-09 5.270e-09 -8.270 -8.278 -0.008
H2O 5.551e+01 1.000e+00 1.744 -0.000 0.000
Al 7.413e-07
Al(OH)2+ 4.905e-07 4.819e-07 -6.309 -6.317 -0.008
AlOH2+ 1.333e-07 1.242e-07 -6.875 -6.906 -0.031
Al(OH)4- 5.279e-08 5.186e-08 -7.277 -7.285 -0.008
Al(OH)3 3.767e-08 3.767e-08 -7.424 -7.424 0.000
Al+3 2.614e-08 2.238e-08 -7.583 -7.650 -0.068
AlSO4+ 8.210e-10 8.066e-10 -9.086 -9.093 -0.008
Al(SO4)2- 3.087e-13 3.033e-13 -12.510 -12.518 -0.008
AlHSO42+ 9.493e-17 8.844e-17 -16.023 -16.053 -0.031
B 2.035e-05
H3BO3 2.034e-05 2.035e-05 -4.692 -4.692 0.000
H2BO3- 8.430e-09 8.282e-09 -8.074 -8.082 -0.008
C(-4) 0.000e+00
CH4 0.000e+00 0.000e+00 -57.660 -57.660 0.000
C(4) 3.211e-04
CO2 2.417e-04 2.417e-04 -3.617 -3.617 0.000
HCO3- 7.927e-05 7.789e-05 -4.101 -4.109 -0.008
CaHCO3+ 4.136e-08 4.064e-08 -7.383 -7.391 -0.008
MgHCO3+ 1.773e-08 1.742e-08 -7.751 -7.759 -0.008
CO32- 2.732e-09 2.546e-09 -8.564 -8.594 -0.031
NaHCO3 5.241e-10 5.241e-10 -9.281 -9.281 0.000
CaCO3 1.738e-10 1.738e-10 -9.760 -9.760 0.000
FeHCO3+ 1.310e-10 1.287e-10 -9.883 -9.890 -0.008
MnHCO3+ 1.189e-10 1.168e-10 -9.925 -9.933 -0.008
MgCO3 4.386e-11 4.386e-11 -10.358 -10.358 0.000
SrHCO3+ 1.084e-11 1.065e-11 -10.965 -10.973 -0.008
MnCO3 3.402e-12 3.403e-12 -11.468 -11.468 0.000
FeCO3 1.009e-12 1.010e-12 -11.996 -11.996 0.000
NaCO3- 4.468e-13 4.390e-13 -12.350 -12.358 -0.008
SrCO3 1.487e-14 1.487e-14 -13.828 -13.828 0.000
Ca 4.791e-05
Ca2+ 4.776e-05 4.451e-05 -4.321 -4.352 -0.031
CaSO4 1.031e-07 1.031e-07 -6.987 -6.987 0.000
CaHCO3+ 4.136e-08 4.064e-08 -7.383 -7.391 -0.008
CaCO3 1.738e-10 1.738e-10 -9.760 -9.760 0.000
CaOH+ 5.837e-12 5.734e-12 -11.234 -11.242 -0.008
CaHSO4+ 7.465e-13 7.334e-13 -12.127 -12.135 -0.008
Cl 2.764e-05
Cl- 2.764e-05 2.716e-05 -4.558 -4.566 -0.008
MnCl+ 1.894e-12 1.861e-12 -11.723 -11.730 -0.008
FeCl+ 6.306e-13 6.196e-13 -12.200 -12.208 -0.008
MnCl2 2.206e-17 2.206e-17 -16.656 -16.656 0.000
FeCl2+ 8.950e-21 8.338e-21 -20.048 -20.079 -0.031
MnCl3- 1.680e-22 1.650e-22 -21.775 -21.782 -0.008
FeCl2+ 1.210e-24 1.188e-24 -23.917 -23.925 -0.008
FeCl3 3.227e-30 3.227e-30 -29.491 -29.491 0.000
Fe(2) 1.790e-08
Fe2+ 1.773e-08 1.653e-08 -7.751 -7.782 -0.030
FeHCO3+ 1.310e-10 1.287e-10 -9.883 -9.890 -0.008
FeSO4 3.260e-11 3.261e-11 -10.487 -10.487 0.000
FeOH+ 2.824e-12 2.774e-12 -11.549 -11.557 -0.008
FeCO3 1.009e-12 1.010e-12 -11.996 -11.996 0.000
FeCl+ 6.306e-13 6.196e-13 -12.200 -12.208 -0.008
FeHSO4+ 2.772e-16 2.723e-16 -15.557 -15.565 -0.008
Fe(3) 1.038e-11
Fe(OH)2+ 9.574e-12 9.406e-12 -11.019 -11.027 -0.008
Fe(OH)3 7.535e-13 7.536e-13 -12.123 -12.123 0.000
FeOH2+ 4.764e-14 4.438e-14 -13.322 -13.353 -0.031
Fe(OH)4- 4.426e-16 4.349e-16 -15.354 -15.362 -0.008
Fe3+ 1.396e-17 1.194e-17 -16.855 -16.923 -0.068
FeSO4+ 1.450e-18 1.425e-18 -17.839 -17.846 -0.008
FeCl2+ 8.950e-21 8.338e-21 -20.048 -20.079 -0.031
Fe(SO4)2- 3.787e-22 3.721e-22 -21.422 -21.429 -0.008
FeHSO42+ 5.307e-24 4.944e-24 -23.275 -23.306 -0.031
FeCl2+ 1.210e-24 1.188e-24 -23.917 -23.925 -0.008
Fe2(OH)24+ 8.683e-26 6.540e-26 -25.061 -25.184 -0.123
FeCl3 3.227e-30 3.227e-30 -29.491 -29.491 0.000
Fe3(OH)45+ 3.199e-34 2.055e-34 -33.495 -33.687 -0.192
H(0) 2.378e-23
H2 1.189e-23 1.189e-23 -22.925 -22.925 0.000
K 1.100e-05
K+ 1.100e-05 1.080e-05 -4.959 -4.966 -0.008
KSO4- 8.608e-10 8.457e-10 -9.065 -9.073 -0.008
KOH 2.907e-14 2.908e-14 -13.536 -13.536 0.000
Mg 2.098e-05
Mg2+ 2.091e-05 1.949e-05 -4.680 -4.710 -0.030
MgSO4 4.880e-08 4.881e-08 -7.312 -7.312 0.000
MgHCO3+ 1.773e-08 1.742e-08 -7.751 -7.759 -0.008
MgCO3 4.386e-11 4.386e-11 -10.358 -10.358 0.000
MgOH+ 3.533e-11 3.471e-11 -10.452 -10.460 -0.008
Mn(2) 1.820e-08
Mn2+ 1.805e-08 1.682e-08 -7.744 -7.774 -0.030
MnHCO3+ 1.189e-10 1.168e-10 -9.925 -9.933 -0.008
MnSO4 3.305e-11 3.306e-11 -10.481 -10.481 0.000
MnCO3 3.402e-12 3.403e-12 -11.468 -11.468 0.000
MnCl+ 1.894e-12 1.861e-12 -11.723 -11.730 -0.008
MnOH+ 2.257e-13 2.218e-13 -12.646 -12.654 -0.008
Mn(NO3)2 3.477e-17 3.477e-17 -16.459 -16.459 0.000
MnCl2 2.206e-17 2.206e-17 -16.656 -16.656 0.000
MnCl3- 1.680e-22 1.650e-22 -21.775 -21.782 -0.008
Mn(3) 2.901e-30
Mn3+ 2.901e-30 2.474e-30 -29.537 -29.607 -0.069
N(5) 2.306e-05
NO3- 2.306e-05 2.266e-05 -4.637 -4.645 -0.008
Mn(NO3)2 3.477e-17 3.477e-17 -16.459 -16.459 0.000
Na 1.218e-05
Na+ 1.218e-05 1.197e-05 -4.914 -4.922 -0.008
NaSO4- 7.196e-10 7.070e-10 -9.143 -9.151 -0.008
NaHCO3 5.241e-10 5.241e-10 -9.281 -9.281 0.000
NaCO3- 4.468e-13 4.390e-13 -12.350 -12.358 -0.008
NaOH 6.136e-14 6.136e-14 -13.212 -13.212 0.000
O(0) 0.000e+00
O2 0.000e+00 0.000e+00 -48.205 -48.205 0.000
S(6) 1.322e-05
SO42- 1.307e-05 1.218e-05 -4.884 -4.915 -0.031
CaSO4 1.031e-07 1.031e-07 -6.987 -6.987 0.000
MgSO4 4.880e-08 4.881e-08 -7.312 -7.312 0.000
HSO4- 1.395e-09 1.370e-09 -8.855 -8.863 -0.008
KSO4- 8.608e-10 8.457e-10 -9.065 -9.073 -0.008
AlSO4+ 8.210e-10 8.066e-10 -9.086 -9.093 -0.008
NaSO4- 7.196e-10 7.070e-10 -9.143 -9.151 -0.008
MnSO4 3.305e-11 3.306e-11 -10.481 -10.481 0.000
FeSO4 3.260e-11 3.261e-11 -10.487 -10.487 0.000
SrSO4 2.371e-11 2.371e-11 -10.625 -10.625 0.000
CaHSO4+ 7.465e-13 7.334e-13 -12.127 -12.135 -0.008
Al(SO4)2- 3.087e-13 3.033e-13 -12.510 -12.518 -0.008
FeHSO4+ 2.772e-16 2.723e-16 -15.557 -15.565 -0.008
AlHSO42+ 9.493e-17 8.844e-17 -16.023 -16.053 -0.031
FeSO4+ 1.450e-18 1.425e-18 -17.839 -17.846 -0.008
Fe(SO4)2- 3.787e-22 3.721e-22 -21.422 -21.429 -0.008
FeHSO42+ 5.307e-24 4.944e-24 -23.275 -23.306 -0.031
Si 2.791e-04
H4SiO4 2.791e-04 2.791e-04 -3.554 -3.554 0.000
H3SiO4- 2.717e-08 2.669e-08 -7.566 -7.574 -0.008
H2SiO4-2 1.082e-15 1.008e-15 -14.966 -14.997 -0.031
Sr 1.141e-08
Sr2+ 1.138e-08 1.061e-08 -7.944 -7.974 -0.031
SrSO4 2.371e-11 2.371e-11 -10.625 -10.625 0.000
SrHCO3+ 1.084e-11 1.065e-11 -10.965 -10.973 -0.008
SrCO3 1.487e-14 1.487e-14 -13.828 -13.828 0.000
SrOH+ 4.297e-16 4.222e-16 -15.367 -15.374 -0.008
-----------------------Saturation indices-------------- BLOC 3.4------
Phase SI log IAP log KT
Al(OH)3(a) -1.11 10.02 11.13 Al(OH)3
Albite -4.54 -22.87 -18.33 NaAlSi3O8
Alunite -1.63 -2.41 -0.77 KAl3(SO4)2(OH)6
Anhydrite -4.92 -9.27 -4.34 CaSO4
Anorthite -6.17 -26.03 -19.86 CaAl2Si2O8
Aragonite -4.64 -12.95 -8.31 CaCO3
Ca-Montmorillonite 3.24 -42.52 -45.76 Ca0.165Al2.33Si3.67O10(OH)2
Calcite -4.49 -12.95 -8.45 CaCO3
Celestite -6.27 -12.89 -6.62 SrSO4
CH4(g) -54.86 -57.66 -2.80 CH4
Chalcedony 0.06 -3.55 -3.61 SiO2
Chlorite(14A) -25.55 44.73 70.27 Mg5Al2Si3O10(OH)8
Chrysotile -18.73 14.10 32.83 Mg3Si2O5(OH)4
CO2(g) -2.21 -3.62 -1.41 CO2
Dolomite -9.28 -26.25 -16.97 CaMg(CO3)2
Fe(OH)3(a) -4.14 0.75 4.89 Fe(OH)3
Gibbsite 1.62 10.02 8.40 Al(OH)3
Goethite 1.57 0.75 -0.82 FeOOH
Gypsum -4.68 -9.27 -4.58 CaSO4:2H2O
H2(g) -19.73 24.10 43.83 H2
H2O(g) -1.64 -0.00 1.64 H2O
Halite -11.06 -9.49 1.57 NaCl
Hausmannite -30.49 31.80 62.29 Mn3O4
Hematite 5.12 1.49 -3.62 Fe2O3
Illite 0.53 -40.42 -40.95 K0.6Mg0.25Al2.3Si3.5O10(OH)2
Jarosite-K -21.41 -30.22 -8.82 KFe3(SO4)2(OH)6
K-feldspar -1.96 -22.91 -20.96 KAlSi3O8
K-mica 6.87 20.32 13.45 KAl3Si3O10(OH)2
Kaolinite 5.05 12.93 7.88 Al2Si2O5(OH)4
Manganite -11.44 13.90 25.34 MnOOH
Melanterite -10.42 -12.70 -2.27 FeSO4:7H2O
O2(g) -45.35 -48.21 -2.85 O2
Pyrochroite -11.19 4.01 15.20 Mn(OH)2
Pyrolusite -18.41 23.79 42.19 MnO2:H2O
Quartz 0.48 -3.55 -4.04 SiO2
Rhodochrosite -5.26 -16.37 -11.11 MnCO3
Sepiolite -12.42 3.48 15.89 Mg2Si3O7.5OH:3H2O
Sepiolite(d) -15.18 3.48 18.66 Mg2Si3O7.5OH:3H2O
Siderite -5.52 -16.38 -10.86 FeCO3
SiO2(a) -0.80 -3.55 -2.75 SiO2
Strontianite -7.30 -16.57 -9.27 SrCO3
Talc -14.99 6.99 21.98 Mg3Si4O10(OH)2
-----------------------------------------
Beginning of batch-reaction calculations. (BLOC 4)
-----------------------------------------
Reaction step 1.
Using solution 1.
Using pure phase assemblage 1.
---------------------Phase assemblage--------------------- (BLOC4.1)---
Moles in assemblage
Phase SI log IAP log KT Initial Final Delta
Al(OH)3(a) -2.16 8.97 11.13 1.000e+01 1.000e+01 2.674e-04
Albite -1.86 -20.19 -18.33 1.000e+01 1.000e+01 -2.667e-04
Calcite -0.53 -8.98 -8.45 1.000e+01 9.998e+00 -1.578e-03
CO2(g) -1.04 -2.45 -1.41 1.000e+01 9.994e+00 -6.240e-03
Dolomite -1.58 -18.55 -16.97 1.000e+01 9.999e+00 -5.376e-04
-----------------------Solution composition--------------(BLOC4.2) --
Elements Output(Moles) Pr 15(Moles)
Al3+ 3.739e-08 3.708e-08
B3+ 2.035e-05 9.256e-08
C4+ 9.215e-03 9.276e-03
Ca2+ 2.164e-03 2.203e-03
Cl- 2.765e-05 9.314e-05
Fe2+ 1.791e-08 1.792e-08
K+ 1.100e-05 2.303e-05
Mg2+ 5.586e-04 5.544e-04
Mn2+ 1.820e-08 1.821e-08
N5+ 2.306e-05 1.055e-04
Na+ 2.789e-04 4.139e-04
S3+ (6) 1.322e-05 3.281e-05
Si4+ 1.079e-03 9.512e-04
Sr2+ 1.141e-08 2.741e-06
--------------Description of solution-------------- (BLOC4.3) -------
pH = 6.532 Charge balance
pe = 13.188 Adjusted to redox equilibrium
Activity of water = 1.000
Ionic strength = 8.219e-03
Mass of water (kg) = 9.999e-01
Total alkalinity (eq/kg) = 5.651e-03
Total CO2 (mol/kg) = 9.215e-03
Temperature (deg C) = 20.000
Electrical balance (eq) = 6.611e-06
Percent error (Cat-|An|)/(Cat+|An|) = 0.06
Iterations = 19
Total H = 1.110129e+02
Total O = 5.553001e+01
-------------------Distribution of species---------------- (BLOC4.4) ----
Log Log Log
Species Molality Activity Molality Activity Gamma
H+ 3.193e-07 2.938e-07 -6.496 -6.532 -0.036
OH- 2.542e-08 2.310e-08 -7.595 -7.636 -0.041
H2O 5.551e+01 9.998e-01 1.744 -0.000 0.000
Al 3.739e-08
Al(OH)4- 2.233e-08 2.033e-08 -7.651 -7.692 -0.041
Al(OH)2+ 1.080e-08 9.833e-09 -7.967 -8.007 -0.041
Al(OH)3 3.363e-09 3.370e-09 -8.473 -8.472 0.001
Al(OH)+2 8.412e-10 5.780e-10 -9.075 -9.238 -0.163
Al+3 5.024e-11 2.376e-11 -10.299 -10.624 -0.325
AlSO4+ 5.689e-13 5.179e-13 -12.245 -12.286 -0.041
Al(SO4)2- 1.294e-16 1.178e-16 -15.888 -15.929 -0.041
Al(HSO4)2+ 1.885e-20 1.295e-20 -19.725 -19.888 -0.163
B 2.035e-05
H3BO3 2.031e-05 2.035e-05 -4.692 -4.691 0.001
H2BO3- 3.990e-08 3.633e-08 -7.399 -7.440 -0.041
C(-4) 0.000e+00
CH4 0.000e+00 0.000e+00 -135.128 -135.127 0.001
C4+(4) 9.215e-03
HCO3- 5.528e-03 5.048e-03 -2.257 -2.297 -0.039
CO2 3.567e-03 3.574e-03 -2.448 -2.447 0.001
Ca(HCO3)+ 9.305e-05 8.497e-05 -4.031 -4.071 -0.039
Mg(HCO3)+ 2.373e-05 2.161e-05 -4.625 -4.665 -0.041
CaCO3 1.591e-06 1.594e-06 -5.798 -5.798 0.001
CO32- 1.041e-06 7.235e-07 -5.983 -6.141 -0.158
NaHCO3 7.182e-07 7.19e-07 -6.144 -6.143 0.001
MgCO3 2.381e-07 2.385e-07 -6.623 -6.622 0.001
MnHCO3+ 4.536e-09 4.129e-09 -8.343 -8.384 -0.041
NaCO3- 2.903e-09 2.643e-09 -8.537 -8.578 -0.041
SrHCO3+ 5.387e-10 4.919e-10 -9.269 -9.308 -0.039
MnCO3 5.265e-10 5.275e-10 -9.279 -9.278 0.001
SrCO3 3.007e-12 3.012e-12 -11.522 -11.521 0.001
FeHCO3+ 4.060e-16 3.696e-16 -15.391 -15.432 -0.041
FeCO3 1.269e-17 1.271e-17 -16.897 -16.896 0.001
Ca 2.164e-03
Ca2+ 2.067e-03 1.436e-03 -2.685 -2.843 -0.158
CaHCO3+ 9.305e-05 8.497e-05 -4.031 -4.071 -0.039
CaSO4 2.009e-06 2.012e-06 -5.697 -5.696 0.001
CaCO3 1.591e-06 1.594e-06 -5.798 -5.798 0.001
CaOH+ 8.909e-10 8.111e-10 -9.050 -9.091 -0.041
CaHSO4+ 3.585e-12 3.264e-12 -11.445 -11.486 -0.041
Cl 2.765e-05
Cl- 2.765e-05 2.513e-05 -4.558 -4.600 -0.041
MnCl+ 1.032e-12 9.399e-13 -11.986 -12.027 -0.041
MnCl2 1.029e-17 1.031e-17 -16.987 -16.987 0.001
FeCl+2 7.667e-19 5.268e-19 -18.115 -18.278 -0.163
FeCl+ 2.791e-20 2.541e-20 -19.554 -19.595 -0.041
MnCl3- 7.841e-23 7.139e-23 -22.106 -22.146 -0.041
FeCl2+ 7.633e-23 6.949e-23 -22.117 -22.158 -0.041
FeCl3 1.743e-28 1.747e-28 -27.759 -27.758 0.001
Fe(2) 1.468e-15
Fe2+ 1.047e-15 7.323e-16 -14.980 -15.135 -0.155
FeHCO3+ 4.060e-16 3.696e-16 -15.391 -15.432 -0.041
FeCO3 1.269e-17 1.271e-17 -16.897 -16.896 0.001
FeSO4 8.721e-19 8.737e-19 -18.059 -18.059 0.001
FeOH+ 5.919e-19 5.389e-19 -18.228 -18.269 -0.041
FeCl+ 2.791e-20 2.541e-20 -19.554 -19.595 -0.041
FeHSO4+ 1.828e-24 1.664e-24 -23.738 -23.779 -0.041
Fe(HS)2 0.000e+00 0.000e+00 -276.226 -276.225 0.001
Fe(HS)3- 0.000e+00 0.000e+00 -409.167 -409.208 -0.041
Fe(3) 1.791e-08
Fe(OH)2+ 1.355e-08 1.234e-08 -7.868 -7.909 -0.041
Fe(OH)3 4.325e-09 4.333e-09 -8.364 -8.363 0.001
Fe(OH)2+ 1.933e-11 1.328e-11 -10.714 -10.877 -0.163
Fe(OH)4- 1.204e-11 1.096e-11 -10.919 -10.960 -0.041
Fe3+ 1.724e-15 8.154e-16 -14.763 -15.089 -0.325
FeSO4+ 6.461e-17 5.883e-17 -16.190 -16.230 -0.041
FeCl2+ 7.667e-19 5.268e-19 -18.115 -18.278 -0.163
Fe2(OH)24+ 2.627e-20 5.857e-21 -19.581 -20.232 -0.652
Fe(SO4)2- 1.020e-20 9.290e-21 -19.991 -20.032 -0.041
FeCl2+ 7.633e-23 6.949e-23 -22.117 -22.158 -0.041
FeHSO42+ 6.775e-23 4.655e-23 -22.169 -22.332 -0.163
Fe3(OH)45+ 2.519e-25 2.414e-26 -24.599 -25.617 -1.018
FeCl3 1.743e-28 1.747e-28 -27.759 -27.758 0.001
H(0) 0.000e+00
H2 0.000e+00 0.000e+00 -42.585 -42.584 0.001
K 1.100e-05
K+ 1.100e-05 9.999e-06 -4.959 -5.000 -0.041
KSO4- 5.200e-10 4.734e-10 -9.284 -9.325 -0.041
KOH 1.177e-13 1.180e-13 -12.929 -12.928 0.001
Mg 5.586e-04
Mg2+ 5.341e-04 3.731e-04 -3.272 -3.428 -0.156
MgHCO3+ 2.373e-05 2.161e-05 -4.625 -4.665 -0.041
MgSO4 5.639e-07 5.650e-07 -6.249 -6.248 0.001
MgCO3 2.381e-07 2.385e-07 -6.623 -6.622 0.001
MgOH+ 3.199e-09 2.912e-09 -8.495 -8.536 -0.041
Mn(2) 1.820e-08
Mn2+ 1.313e-08 9.179e-09 -7.882 -8.037 -0.155
MnHCO3+ 4.536e-09 4.129e-09 -8.343 -8.384 -0.041
MnCO3 5.265e-10 5.275e-10 -9.279 -9.278 0.001
MnSO4 1.089e-11 1.091e-11 -10.963 -10.962 0.001
MnCl+ 1.032e-12 9.399e-13 -11.986 -12.027 -0.041
MnOH+ 5.826e-13 5.304e-13 -12.235 -12.275 -0.041
Mn(NO3)2 1.616e-17 1.620e-17 -16.791 -16.791 0.001
MnCl2 1.029e-17 1.031e-17 -16.987 -16.987 0.001
MnCl3- 7.841e-23 7.139e-23 -22.106 -22.146 -0.041
Mn(3) 4.838e-21
Mn3+ 4.838e-21 2.080e-21 -20.315 -20.682 -0.367
N(0) 4.616e-09
N2 2.308e-09 2.312e-09 -8.637 -8.636 0.001
N(3) 1.098e-15
NO2- 1.098e-15 9.970e-16 -14.959 -15.001 -0.042
N(5) 2.306e-05
NO3- 2.306e-05 2.093e-05 -4.637 -4.679 -0.042
Mn(NO3)2 1.616e-17 1.620e-17 -16.791 -16.791 0.001
Na 2.789e-04
Na+ 2.782e-04 2.535e-04 -3.556 -3.596 -0.040
NaHCO3 7.182e-07 7.196e-07 -6.144 -6.143 0.001
NaSO4- 9.949e-09 9.059e-09 -8.002 -8.043 -0.041
NaCO3- 2.903e-09 2.643e-09 -8.537 -8.578 -0.041
NaOH 5.688e-12 5.699e-12 -11.245 -11.244 0.001
O(0) 2.591e-09
O2 1.296e-09 1.298e-09 -8.888 -8.887 0.001
S(-2) 0.000e+00
H2S 0.000e+00 0.000e+00 -134.543 -134.542 0.001
HS- 0.000e+00 0.000e+00 -134.978 -135.020 -0.041
S2- 0.000e+00 0.000e+00 -141.398 -141.557 -0.159
Fe(HS)2 0.000e+00 0.000e+00 -276.226 -276.225 0.001
Fe(HS)3- 0.000e+00 0.000e+00 -409.167 -409.208 -0.041
S(6) 1.322e-05
SO42- 1.064e-05 7.363e-06 -4.973 -5.133 -0.160
CaSO4 2.009e-06 2.012e-06 -5.697 -5.696 0.001
MgSO4 5.639e-07 5.650e-07 -6.249 -6.248 0.001
NaSO4- 9.949e-09 9.059e-09 -8.002 -8.043 -0.041
KSO4- 5.200e-10 4.734e-10 -9.284 -9.325 -0.041
HSO4- 2.076e-10 1.890e-10 -9.683 -9.723 -0.041
MnSO4 1.089e-11 1.091e-11 -10.963 -10.962 0.001
SrSO4 1.020e-11 1.022e-11 -10.991 -10.990 0.001
CaHSO4+ 3.585e-12 3.264e-12 -11.445 -11.486 -0.041
AlSO4+ 5.689e-13 5.179e-13 -12.245 -12.286 -0.041
Al(SO4)2- 1.294e-16 1.178e-16 -15.888 -15.929 -0.041
FeSO4+ 6.461e-17 5.883e-17 -16.190 -16.230 -0.041
FeSO4 8.721e-19 8.737e-19 -18.059 -18.059 0.001
AlHSO4+2 1.885e-20 1.295e-20 -19.725 -19.888 -0.163
Fe(SO4)2- 1.020e-20 9.290e-21 -19.991 -20.032 -0.041
FeHSO4+2 6.775e-23 4.655e-23 -22.169 -22.332 -0.163
FeHSO4+ 1.828e-24 1.664e-24 -23.738 -23.779 -0.041
Si 1.079e-03
H4SiO4 1.079e-03 1.081e-03 -2.967 -2.966 0.001
H3SiO4- 4.978e-07 4.532e-07 -6.303 -6.344 -0.041
H2SiO42- 1.092e-13 7.502e-14 -12.962 -13.125 -0.163
Sr 1.141e-08
Sr2+ 1.086e-08 7.560e-09 -7.964 -8.121 -0.157
SrHCO3+ 5.387e-10 4.919e-10 -9.269 -9.308 -0.039
SrSO4 1.020e-11 1.022e-11 -10.991 -10.990 0.001
SrCO3 3.007e-12 3.012e-12 -11.522 -11.521 0.001
SrOH+ 1.446e-15 1.319e-15 -14.840 -14.880 -0.040
--------------------Saturation indices--------------(BLOC4.5) --------
Phase SI log IAP log KT
Al(OH)3(a) -2.16 8.97 11.13 Al(OH)3
Albite -1.86 -20.19 -18.33 NaAlSi3O8
Alunite -7.18 -7.95 -0.77 KAl3(SO4)2(OH)6
Anhydrite -3.63 -7.98 -4.34 CaSO4
Anorthite -4.30 -24.16 -19.86 CaAl2Si2O8
Aragonite -0.68 -8.98 -8.31 CaCO3
Ca-Montmorillonite 3.42 -42.34 -45.76 Ca0.165Al2.33Si3.67O10(OH)2
Calcite -0.53 -8.98 -8.45 CaCO3
Celestite -6.63 -13.25 -6.62 SrSO4
CH4(g) -132.33 -135.13 -2.80 CH4
Chalcedony 0.64 -2.97 -3.61 SiO2
Chlorite(14A) -13.05 57.22 70.27 Mg5Al2Si3O10(OH)8
Chrysotile -9.86 22.97 32.83 Mg3Si2O5(OH)4
CO2(g) -1.04 -2.45 -1.41 CO2
Dolomite -1.58 -18.55 -16.97 CaMg(CO3)2
Fe(OH)3(a) -0.38 4.51 4.89 Fe(OH)3
FeS(ppt) -139.71 -143.62 -3.92 FeS
Gibbsite 0.58 8.97 8.40 Al(OH)3
Goethite 5.33 4.51 -0.82 FeOOH
Gypsum -3.39 -7.98 -4.58 CaSO4:2H2O
H2(g) -39.39 4.44 43.83 H2
H2O(g) -1.64 -0.00 1.64 H2O
H2S(g) -133.55 -141.55 -8.00 H2S
Halite -9.77 -8.20 1.57 NaCl
Hausmannite -7.77 54.52 62.29 Mn3O4
Hematite 12.64 9.01 -3.62 Fe2O3
Illite 1.18 -39.77 -40.95 K0.6Mg0.25Al2.3Si3.5O10(OH)2
Jarosite-K -12.52 -21.34 -8.82 KFe3(SO4)2(OH)6
K-feldspar -0.63 -21.59 -20.96 KAlSi3O8
K-mica 6.10 19.55 13.45 KAl3Si3O10(OH)2
Kaolinite 4.13 12.01 7.88 Al2Si2O5(OH)4
Mackinawite -138.98 -143.62 -4.65 FeS
Manganite -0.59 24.75 25.34 MnOOH
Melanterite -18.00 -20.27 -2.27 FeSO4:7H2O
N2(g) -5.49 -8.64 -3.14 N2
O2(g) -6.03 -8.89 -2.85 O2
Pyrite -227.12 -245.74 -18.62 FeS2
Pyrochroite -10.17 5.03 15.20 Mn(OH)2
Pyrolusite 2.27 44.47 42.19 MnO2:H2O
Quartz 1.07 -2.97 -4.04 SiO2
Rhodochrosite -3.07 -14.18 -11.11 MnCO3
Sepiolite -5.52 10.37 15.89 Mg2Si3O7.5OH:3H2O
Sepiolite(d) -8.29 10.37 18.66 Mg2Si3O7.5OH:3H2O
Siderite -10.42 -21.28 -10.86 FeCO3
SiO2(a) -0.21 -2.97 -2.75 SiO2
Strontianite -4.99 -14.26 -9.27 SrCO3
Sulfur -100.10 -95.10 5.00 S
Talc -4.94 17.04 21.98 Mg3Si4O10(OH)2
------------------
End of simulation.
Reading input data for simulation 2.
End of run.
No memory leaks
----------