Evaluation of Some Radioactive Materials and Heavy Metals in Marine Environment of Alexandria Coastline, Egypt ()
1. Introduction
Pollution is the most serious of all environmental problems and posses a major threat to the health and well- being of millions of people and global ecosystems. In recent years, pollution becomes a paramount problem with increasing the human activities. Natural and artificial radionuclides have attained great attention of many researchers in the last three decades especially after Chernobyl radiation accident in 1986. Many authors investigated the Ocean waters [1] and marine sediments [2] - [4] . The sources of radiation in water are also the sources in the case of sediment. The most dominant radionuclides are 40K and also decay products of the 238U and 232Th series [5] . Most of radionuclides have a low solubility in water and tend to be sorbed onto the particulate matter, therefore they accumulate in sediments. Fine sediments, with their large surface area, tend to sorb more than coarse sediments. Thus, sea water has a radioactivity of about 12.6 Bq∙kg−1, while marine sand has a radioactivity of 200 - 400 Bq∙kg−1, and mud 700 - 1000 Bq∙kg−1, in parts of the world [5] . The pollution of marine ecosystems by heavy metals is a world-wide problem. Generally, the coastal industries appear to discharge wastes directly into the seas environments with little or no treatment. Mediterranean Sea is a semi-closed basin connected to the Atlantic Ocean through the narrow and shallow Strait of Gibraltar [6] . So the ecosystem of the Mediterranean Sea affected by chemical pollutant resulted from the human activities. The contamination levels of the aquatic environment by heavy metals can be estimated by analyzing its water and sediments. Heavy metals are considered a major contaminant in coastal and marine environments worldwide [7] . They pose a serious threat to human health, living organisms and natural ecosystems because of their toxicity, persistence and bioaccumulation characteristics [8] . Many heavy metal ions are known to be toxic or carcinogenic to human [9] . Metals such as copper, zinc and manganese are essential metals since they play important roles in biological systems [10] , whereas non-essential metals such as Pb, and Cd are usually potent toxins and their bioaccumulation in tissues leads to intoxication, decreased fertility, cellular and tissue damage, cell death and dysfunction of a variety of organs [11] [12] . Heavy metals can contribute in degradation of marine ecosystems by reducing species diversity and through accumulation of metals in living organisms [13] .
The coastal states of the Mediterranean Sea do their efforts to protect the nature of the sea. Egypt has about 1050 km of coastline along the Mediterranean Sea proper, which is of great environmental, economical and recreational value. Some of the Egyptian coastal areas of the Mediterranean Sea (especially in front of the large cities) receive different types of pollution sources, so the study area located between 31˚08'11''N into 31˚21'18''N and 29˚50'02''E into 30˚18'20''E. The present work is carried out to obtain quantitative information on some natural radioactive materials and heavy metals in water and sediment samples along the Alexandria Coastline, as a step to construct the baseline map of the background radioactivity level in the Egyptian environment and also as a base data to assess the future physicochemical changes of coastal surface water and sediment in the study area.
2. Materials and Methods
2.1. Study Area
The investigated area (110 km along) expanded along the coast of Alexandria, from Al-Max 31˚08'11''N to 29˚5'02''E in west to Abu-Qir 31˚17'20''N to 30˚08'23''E in east and stretches to Rashid coast city 31˚21'18''N to 30˚18'20''E.
2.2. Sampling and Sample Preparation
Collected samples in the coastline study area occurred during spring 2012. Five representative surficial shore sediment samples were collected using the template method at the same sites and time of seawater samples. An area of about 25 ´ 25 cm2 up to a depth of 5 cm was cut out using the stainless steel template for guidance [14] . The collected shore sediment samples were transferred to labelled polyethylene bags, closed and transported to the laboratory for preparation and chemical analysis.
The shore sediment samples were air-dried at room temperature for a week. And also were dried in an oven at 80˚C (for 48 h) till constant dry weight was obtained, crushed and homogenized. Then milled and sieved through 0.4 mm mesh sieves and stored for further analysis. Water samples, 5 liter of each, were collected using the water sampler. They were collected in polyethylene containers. Then, the samples were acidified with Nitric acid to pH lower than 2 to avoid micro-organisms growth and to minimize water-walls interaction. The samples were stored for radioactivity measurements and chemical analysis [14] . The total dissolved solid (TDS), salinity, pH and temperature (˚C) were measured in the field after sampling. These measurements carried out according to [15] .
2.3. Heavy Metals Measurements in Sediment and Water Samples
Atomic absorption Spectrophotometer (AAS) is a simple and well available technique for the determinations of heavy metals in the water and soil samples. Heavy metals in sediments were determined according to [16] . The sediments were digested with 5:1 mixture of HF and HClO4 acids. 1 g (dry weight) sample was digested by 2 ml HClO4 and 10 ml HF to near dryness, subsequently a second addition of 1 ml of HClO4 and 10 ml of HF and evaporated to near dryness. Finally, 1 ml of HClO4 alone was added and the sample was evaporated until the appearance of white fumes. The residue was dissolved in 12 N HCl and diluted to 25 ml with de-ionized water. The metal ions were determined by Atomic Absorption Spectrophotometer, Perkin Elmer model Aanalyst 100 which is manufactured in USA. The results obtained were determined according to [17] .
2.4. Radioactivity Measurements for Sediment and Water Samples
Radioactivity Measurements for Sediment Samples
1) Sample preparation
The homogenized sediment samples were packed in a 250 ml plastic container to its full volume with uniform mass. These containers were shielded to ensure that all daughter products of Uranium and Thorium, in particular radon gas formed, do not escape. The net weight of the sample was determined before counting. These samples were then stored for 30 - 40 days before counting to reach radioactive equilibrium.
2) Radioactivity measurements
The activity concentration of the natural radioactivity 238U, 232Th and 40K in the investigated samples were determined using a high-resolution HPGe γ-spectrometry system with 30% counting efficiency. This was performed by taking 250 cm3 counting vials filled up to a height of 7 cm, which correspond to 170 cm3. The measurement duration was up to 80,000 sec and were carried out in the Laboratory of Egyptian Nuclear and Radiological Regulatory Authority. The obtained spectra were analyzed. The determination of the presence of radionuclides and calculation of their activities were based on the following gamma-ray transitions (in keV): the 226Ra activities (or 238U activities for samples assumed to be in radioactive equilibrium) were estimated from 234Th (92.38 keV, 5.6%), while γ-energies of 214Pb (351.9 keV, 35.8%) and 214Bi (609.3, 45%), 1764.5 keV, 17%) and 226Ra (185.99 KeV, 3.5%) were used to estimate the concentration of 226Ra. The Gamma-ray energies of 212Pb (238.6 keV, 45%), and 228Ac (338.4 keV, 12.3%), (911.07 keV, 29%), (968.90 keV, 17%) were used to estimate the concentration of 232Th. The activity concentrations of 40K were measured directly by its own gamma rays (1460.8 keV, 10.7%). In order to determine the background distribution due to naturally occurring radionuclides in the environment around the detector, an empty polystyrene container was counted in the same manner as the samples. The activity concentrations were calculated after measurement and subtraction of the background. The activities were determined from measuring their respective decay daughters. The activity concentrations were calculated from the intensity of each line taking into account the mass of the sample, the branching ratios of the γ-decay, the time of counting and the efficiencies of the detector [18] [19] . The activity concentrations of the investigated samples were calculated from Equation (1):
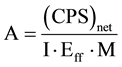 (1)
(1)
where A is the activity concentration in Bq/kg, (CPS)net is the (count per second) and equals
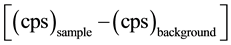 , I is the intensity of the γ-line in a radionuclide, Eff is the measured efficiency for
, I is the intensity of the γ-line in a radionuclide, Eff is the measured efficiency for
each γ-line observed and M is the mass of the sample in kilograms. The correction for the contribution of 232Th via its daughter nuclide 228Ac (1459.2 keV peak) to the 1460.8 keV peak of 40K was made according to following formula [20] .
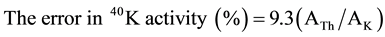 (2)
(2)
where: ATh and AK are the activity concentration of 232Th and 40K, respectively, in Bq∙kg−1.
3. Results and Discussion
Physical characteristics of water samples at different locations of studied area Table 1 represents some physical characteristics of water samples at different locations of studied area. It is clear that the total dissolved solids (TDS) in water samples ranged from 33,000 mg/l to 42,000 mg/l, the salinity is fluctuated pattern and ranged from 37.9% to 40.5% with an average of 39.02% and the marine water is slightly alkaline (pH ranged from 7.6 to 7.9).
3.1. Heavy Metals in Water
The results corresponding to the seawater analysis of the different samples are shown in Table 2 and Figure 1. In general, the analyzed heavy metals showed increasing concentrations of Mn and Zn in all studied locations. It is noted that Cd concentration is the lowest in all locations, while the concentration of Pb, Ni, Cr, Cu and Co ions are in the same level. In general, this may be due to anthropogenic sources, a mixture of contaminated sediments with relatively clean marine sediments and/or the release of metals into the water as a freshwater and seawater combination [21] .
3.2. Heavy Metals in Sediment
The concentrations of heavy metals in the investigated sediment samples of Alexandria coast are shown in Table 3 and Figure 2. It is clear that the most abundant contaminated ions in all locations are Mn, and Zn, Mn > Zn. while Cd is the lowest contaminant ion concentration for all locations. Eastern Harbour has highest concentration of Cr ions; this may be attributed to the effect of ships discharges which used antifouling paints.
![]()
Figure 1. Concentration of some heavy metal ions in marine water at different locations along the study area.
![]()
Table 1. Some physical characteristics of water samples at different locations of studied area.
![]()
Figure 2. Concentration of some heavy metal ions in sediment samples at different locations in study area.
![]()
Table 2. Concentration of some heavy metals in marine samples.
![]()
Table 3. Concentration of some heavy metals in sediment samples and the metal pollution index (MPI).
*Canadian soil quality guidelines (2002) for the protection of environmental and human health; **Persaud et al. (1993).
El-Dekhila has highest concentrations of both Co and Pb; this may be due to the effect of El-Dekhila electric power station which use metal alloys in containers for heating the water steam to produce electricity. Rashid and El-Mex locations have higher concentration of Cu ions. The variety of the concentrations of heavy metals in bulk sediments of Alexandria coast may be attributed to the effect of type and amount of pollutant arrives to it and the characteristic nature of the area [22] .
3.3. Sediment Quality Guidelines
The studied parameters of sediment quality guidelines are the metal pollution index (MPI), the contamination factor (CF), degree of contamination (Cdeg), and Pollution load index (PLI).
3.4. The Metal Pollution Index (MPI)
The degree of heavy metal pollution can be estimated using the metal pollution index (MPI) according to the following formula:
 (4)
(4)
The concentration of a metal is expressed in μg∙g−1; dry weight [23] .
According to the calculated data of the metal pollution index (MPI), (Table 3), it can be concluded that the highest values (50.77 and 41.43) were found at El Dekhela and Abu-Qir locations, respectively. The lowest (29.69) value were found at Eastern Harbour.
3.5. The Contamination Factor (CF)
Contamination factor is an assessment of soil contamination through a reference element in comparison with crustal level. It is an effective tool for monitoring pollution over a period of time and evaluating the pollution of environmental single substances. It is defined according to four categories as follows [24] .
(CF < 1 low contamination factor indicating low contamination; 1 < CF < 3 moderate contamination factor; 3 < CF < 6 considerable contamination factor; CF > 6 very high contamination factor).
Individual contamination factors are calculated based on the following formula:
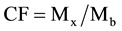 (5)
(5)
where: Mx is the concentration of the target metal and Mb is the concentration of the metal in the selected reference background.
The results of contamination factors (Table 4) indicated that Cd and Cr possess the highest CFs in Eastern Harbour, also El-Dekhila has highest CF of Co. They reflect that sediments in these locations are moderately
![]()
Table 4. Contamination factor (CF), degree of contamination (deg. cont.) and pollution load index (PLI) of some heavy metals in soil sediment samples at studied locations.
contaminated (1 < CF < 3). The rest of metals in all locations are remained within the low contamination level of the sediment (CF < 1).
3.6. Degree of Contamination (Cdeg)
The contamination factor described above is a single element index. The sum of contamination factors for all elements examined represents the contamination degree (Cdeg) of the environment [25] reported that there are four classes of contamination degree. They are Cdeg < 8 low degree of contamination, 8 < Cdeg < 16 moderate degree of contamination, 16 < Cdeg < 32 considerable degree of contamination, and 32 < Cdeg very high degree of contamination.
The sums of contamination factors for all metals examined are listed in Table 4 which shows that the degree of contamination in the investigated sediment samples ranged from 4.38 to 5.38. This indicates that the degree of contamination in the investigated sediment samples in all locations is low. Also it can be seen from the data that the principle contributors among toxic trace metals are Zn, Mn, Ni and Cu ions.
3.7. Pollution Load Index (PLI)
The pollution load index (PLI) of each site was evaluated as indicated by Tomilson et al. (1980) [26] .
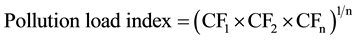 (6)
(6)
where; n is the number of metals and CF is the contamination factor. Contamination can be estimated as follows: (PLI value > 1 polluted; PLI value < 1 unpolluted [23] [27] ). The PLI values calculated for each site were shown in Table 3. It is shown that the PLI for all locations ranged from 0.44 to 0.59. This indicates that all studied sites were found to be low polluted (PLI < 1).
3.8. Activity of the Investigated Radionuclides in Water and Sediment Samples
Table 5 and Figures 3-6 represent the activity of 238U, 232Th and 40K in coastal seawater and sediment samples at different locations along the studied area. It is clear that the measured values for 238U and 232Th in sea water are very low. The 238U values vary from 13.08 Bq∙L−1 at El Mex location to 17.30 Bq∙L−1 at Rashid location. The highest value at Rashid could be attributed to the existence of black sand. Also, the 232Th values are low and varies from 10.32 Bq∙L−1 at Eastern Harbour location to 20.33 at Bq∙L−1 Abu-Qir location. The distribution of 40K
![]()
Figure 3. Activity of 238U and 232Th in marine water at different locations along the study area.
![]()
Figure 4. Activity of 40K in marine water at different locations along the study area.
![]()
Figure 5. Activity of 238U and 232Th in sediment samples at different locations along the study area.
![]()
Table 5. Concentration of 238U, 232Th and 40K in seawater and coastal sediments collected from different locations along the studied area.
![]()
Figure 6. Activity of 40K in sediment samples at different locations along the study area.
in sea water along the locations revealed that, the Al-Mex is poor in 40K (562.04 Bq∙L−1) while Abu-Qir location has a highest activity (1273.02 Bq∙L−1) and it has differences in their patterns. It is clear that the lower 40K has been detected in locations receiving low saline water discharged from inland sources such as Rashid branch and outlets of coastal lakes. Because 40K is an alkali metal, it behaves as a true soluble element and varies in proportion with respect to salinity. So the distribution of 40K in surface seawater and in sediments has differences in their patterns due to the effect of salinity on the behavior of 40K in surface seawater. It is observed that the general trend of natural radionuclides in the sediments decreases in the direction of east locations to the west locations, Figure 6. Also it is noticed that large variation among the radioactivity concentration for different locations. It may be due to geological condition and drainage pattern of the study area.
It is clear that there is a general decreasing trend of 238U from Rashid and Abu-Qir to Al-Max location. This is because the sea wave direction in this region is mostly to the west, which helps in the deposition of the eroded black sand from the Rashid coast which is about 30 km East Abu-Qir.
The 232Th measured values in sediment along the study area more or less have the same trend as 238U. The high values could be explained as due to the presence of black sands, which are enriched in the mineral monazite containing a significant amount of 232Th. The black sands, which is rich in radio-nuclides, supplied by the River Nile during the flooding period in the last periods through the Rashid branch of the Nile. The Mediterranean Sea waves carry part of these black sands to Alexandria beaches westward.
3.9. Determination of the Absorbed Dose Rate, Annual Effective Dose Equivalent, External Hazard Index and Representative Level Index Values
The absorbed dose rate was calculated from the measured activities of 238U, 232Th and 40K in the surface sediment samples using the below formula [28] .
![]() (7)
(7)
where: D is the absorbed dose rate (nGy∙h−1), CU, CTh and CK are the activity concentrations (Bq∙kg−1) of 238U, 232Th and 40K respectively. To estimate the annual effective dose rates, the conversion coefficient from absorbed dose to effective dose, 0.7 Sv∙Gy−1 and outdoor occupancy factor of 0.2 proposed by UNSCEAR, 2000 were used. The effective dose rate in units of mSv∙y−1 was calculated by the following formula:
![]() (8)
(8)
Calculation of hazard indexes (Hex):
The external hazard index, Hex, was calculated using the following formula [29] .
![]() (9)
(9)
An additional hazard index so called representative level index is calculated by using the following formula [23] ,
![]() (10)
(10)
where:![]() ,
, ![]() and
and ![]() are the specific activities (Bq・kg?1) of 238U, 232Th and 40K, respectively. The
are the specific activities (Bq・kg?1) of 238U, 232Th and 40K, respectively. The
value of these indexes must be less than unity in order to keep the radiation hazard insignificant.
Table 6 represents the calculated absorbed gamma dose rate from 33.73 nGy∙h−1 (El-Max) to 67.46 nGy∙h−1 (Rashid), with a mean of 47.02 nGy∙h−1. The mean absorbed dose rate is found to be lower than the world average value (51 nGy∙h−1: UNSCEAR, 2000). The calculated values of annual effective dose rate ranging from 0.041 to 0.083 mSv, with a mean value of 0.058 mSv, which is lower than the world average value of 0.48 mSv (UNSCEAR, 2000). The calculated value of external hazard index ranges from 0.207 to 0.366. The representative level index value being 0.577 to 0.978, with the average of 0.762, which is higher than the world average (0.66 Bq∙kg−1) [30] .
4. Conclusions
The results demonstrate that the main source of radiation along the Alexandria coast is the black sand supplied by the River Nile through the Rashid branch. There is a general decreasing trend of 238U and 232Th from Rashid and Abu-Qir to Al-Max westerly. The distribution of 40K along the Alexandria coast reveals that the Al-Max location is poor in 40K, while Abu-Qir is rich in 40K. There is no pattern for the concentration of 40K in sediments along the studied locations.
The mean absorbed dose rate is found to be lower than the world average value (51 nGy∙h−1). The mean annual effective dose rate is lower than the world average value (0.48 mSv). The average external hazard index value is higher than the world average (0.66 Bq∙kg−1).
Spatial distribution of heavy metals in sediment of the study area revealed that Alexandria coast may be affected by different sources of pollution (sewage and garbage from the Alexandria City and ships awaiting transit area, industrial effluents including oil refineries, fertilizer plant, power stations and other industries).
The index of contamination factor shows that the sediment samples from eastern harbour location are moderately contaminated with chromium and El-Dekhila location is moderately contaminated with cobalt. All other metals analyzed (Pb, Cd, Ni, Mn, Cu and Zn) display low level of contamination in sediment samples. The sum
![]()
Table 6. External hazard index (Hex) values, representative level index (Ir) values, absorbed dose rate and annual effective dose rate for radioactive materials (238U, 233Th and 40K) in different sites in Alexandria coastline.
of contamination factors for all metals examined indicates low degree of contamination in sediment. The degree of contamination (Cdeg) in sediment samples is low in all locations.
NOTES
*Corresponding author.