Degradation of Dyestuff Pollutant Sudan I Using Advanced Oxidation Process ()
1. Introduction
Azo types of dyes [1] , characterized by having an azogroups consisting of two nitrogen atoms (-N=N-) as the chromophore in the molecule. Because of their toxicity and potentially carcinogenic nature, waste waters originating from dyes production and application industries pose a major threat to the surrounding ecosystem and human health. It is known that azodyes are largely non-biodegradable in aerobic conditions due to the strong electron withdrawing character of the azogroup [2] , while in anaerobic conditions [3]; they can be reduced to more hazardous intermediates, such as aromatic amines. Other common commercial processes, such as coagulation/fluoccation and adsorption, do not involve chemical transformations and generally transfer waste components from one phase to another, thus causing secondary loading environment [4] .
Azodyes [5] are an important class of synthetic organic compounds used as colouring agents in the textile, paint, ink and plastic industries. Large amounts of these dyes remain in the effect after the completion of the dyeing process. Consequently, very small amount of dyes in waste water is highly visible. A side from their negative aesthetic effects, certain azodyes and their biotransformation products has been shown to be toxic [6] to aquatic life and mutagenic to humans. Azodyes are stable compounds, difficult to destroy or to be decomposed by common treatment in a biological treating station. Azodyes may be decolorized by cleavage of the azobond, to which the color is associated. The reactive dyes are widely used in the textile industries because of its simple dyeing procedure and good stability during washing process. The textile processing industry is putting a severe burden on the environment through the release of heavily polluted wastewaters. The presence of these dyes in the aqueous ecosystems is the cause of serious environmental and health concerns. Several methods are used to treat textile effluents to achieve decolourization.
Azodyes occupy the leading place in the total volume of commercially produced synthetic dyes and are widely used in various branches of industry. On the one hand, azodye should be resistant to various oxidants (e.g. under laundering conditions) and on the other hand, their degradation in industrial waste water treatment is an urgent problem. Due to the presence of stable chromophoric azogroups (-N=N-), electron withdrawing and electron donating groups [2] , the dyes can be designed to resist chemical or photochemical degradation processes.
The disappearance of the dye is generally followed by spectrophotometry at the absorbance maximum between 450 and 600 nm. Advanced Oxidation Process (AOP) [7] have been proposed and employed for the treatment of hazardous materials in waste water since 1990s. In principle, AOPs are based in the generation of hydroxyl radicals in water; it is highly reactive oxidant, [4] [8] which can oxidize organic compounds, especially unsaturated organic compounds. Among the most promising AOPs for water contaminated by organic molecules, [9] application of the Fenton’s reagent (an aqueous mixture of Fe2+ and H2O2 that produce ·OH radical) [10] [11] stands out due to its high oxidation power, rapid oxidation kinetics, being relatively inexpensive and easy to operate and maintain. However, the application of Fenton’s reagent [12] in the destruction of organic pollutants is limited by the slurry system, because it produces a significant amount of Fe(III)-iron sludge, which requires further separation and disposal.
The expansion of worldwide textile industry has led to an equivalent expansion in the use of such synthetic dyestuffs, and only one among such azo dye categories, is the selected phenyl azo b-naphthol {PAN} or Sudan I [7] . The application of Fenton reagent in cost-effective way is not yet tried in the case of strong azo dye, like Sudan I. Therefore, the present method offers low-cost and moderate source of generating hydroxyl radical, which is the prime oxidizing agent for the decolorization of the selected azo dye. At the same time it results in a rise in environmental pollution due to the contamination of wastewater with the dyestuffs [13] [14] . Therefore, the necessity of degrading it effectively and at the same time ensuring that no harm byproducts are produced, which may unless otherwise, a threat for the natural water resources!
The Ecological and Toxicological Association of the Dyestuffs Manufacturing Industry (ETAD) was inaugurated in 1974 with the goals of minimizing environmental damage, protecting users and consumers and cooperating with government and public concerns in relation to the toxicological impact of their products [15] -[17] . A survey carried out by ETAD showed that of a total of approximately 4000 dyes that had been tested, more than 90% showed lethal dose (LD50) values above 2 × 103 mg/kg, the most toxic being in the group of basic and direct diazo dyes [17] [18] . Thus it appears that exposure to azo dyes does not cause acute toxicity, but with respect to systemic bioavailability, inhalation and contact with the skin by azo dyes is of concern, due to the possible generation of carcinogenic aromatic amines [19] .
The disappearance of the dye is generally followed by spectrophotometry at the absorbance maximum between 450 and 600 nm. Advanced Oxidation Process (AOP) [7] [11] has been proposed and employed for the treatment of hazardous materials in waste water since 1990s. In principle, AOPs are based in the generation of hydroxyl radicals in water; it is highly reactive oxidant which can oxidize organic compounds, especially unsaturated organic compounds. Among the most promising AOPs for water contaminated by organic molecules, application of the Fenton’s reagent {an aqueous mixture of Fe2+ and H2O2 that produce ·OH radical} [11] stands out due to its high oxidation power, rapid oxidation kinetics, being relatively inexpensive and easy to operate and maintain. The advantage of the Fenton reagent is that no energy input is necessary to activate hydrogen peroxide [20] . The role of Fenton reagent for the production of ·OH radical is shown in (Figure 1). Hence, the present work proposes the oxidative degradation of PAN using Fenton reagent in both sun light and uv-light.
2. Material and Methods
5 ml aniline was allowed to dissolve in a mixture of 16 ml concentrated HCl and 20 ml t-distilled water. This mixture was kept in ice cold temperature 0 to 5˚C and mixed the solution of 4 g NaNO3 in 15 ml water with vigorous shaking and controlled the temperature below 5˚C. A solution of 5 g NaOH in 50 ml triply-distilled water in which 8 g of b-naphthol was dissolved and kept in ice cold temperature 0˚C to 5˚C. Mixed the solution drop by drop with continuous stirring at least for half an hour by keeping the temperature constant 0˚C to 5˚C. Filtered and washed with cold water, dried and recrystalized in ethyl alcohol or glacial acetic acid. The solid, which imparted scarlet-red color and this amorphous form, was used for the entire experimentation as phenyl azo b-naphthol (PAN) or (Sudan I).
PAN (10−4 mol∙dm−3) was prepared in chloroform in which various concentrations of Fenton reagent [7] [10] (mixture of ferrous sulfate {10−3, 5 × 10−4, 10−4, 5 × 10−5 mol∙dm−3} and H2O2 {10−2 mol∙dm−3}) were mixed and irradiated in sun light at four different time scales such as (1, 1½, 2 & 2½) hours. The experiments were performed at varying pH (2, 4, 5, 6 & 7). Optical density of the sample at various concentrations of ferrous sulfate were observed using colorimeter (DR/890-portable colorimeter) in varying time. In order to find out the experiments in parallel way by replacing the sun light source as uv-lamp (wavelength 254 nm) (Specrtonics 240 to 365) and irradiated the mixture in 20 minutes.
3. Results and Discussion
The effect of concentration of Fenton reagent on absorbance was studied using both irradiated source such as sunlight and uv-lamp of 254 nm for observing the decolourization efficiency of the substrate at near neutral pH 6. Figure 2 showed 50% reduction in an experimental duration of 2 ½ hours on exposure of sunlight in peek summer and Figure 3, a reduction in 42% observed in just 20 minutes on exposure of uv-light source at 254 nm, simultaneously. The best discoloration is observed in between (1 - 5) × 10−4 mol∙dm−3 in Figure 2 using sunlight and a range of concentrations (2 - 3.7) × 10−4 mol∙dm−3 on exposure of uv-light shown in Figure 3.
![]()
Figure 1. Schematic representations for the coupled Fenton’s and photo-Fenton’s reactions.
![]()
Figure 2. Effect of concentration on absorbance of PAN at pH 6 irradiated (2 ½ hours) by sunlight.
![]()
Figure 3. Effect of concentration of Fenton reagent on absorbance of PAN at pH 6 irradiated 20 min by 254 nm uv-light.
In addition to view of relatively low concentration of Fe2+ in this work, which seemed to be cost-effective, energy saver and environmental friendly, an earlier work reported that catalytic nature of iron [21] helps in enhancing the reaction occurring at lower concentration of iron in solar Fenton’s process.
In Figure 3, discoloration observed maximum at 3 × 10−4 mol∙dm−3 on exposure to 254 nm uv-light. However, all concentations above 3 × 10−4 mol∙dm−3, a decrease in color removal was observed. This inhibitory effect at the higher Fenton concentrations could be explained by an observation (referring to the classical Fenton reaction) that in the absence of organic substrates, excessive ferrous ions (formed from Fe2+/H2O2 process) are the dominant hydroxyl radical’s scavenger through the following reaction (3.1) [22] [23] .
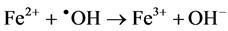 (3.1)
(3.1)
The solution of PAN in chloroform mixed with Fenton reagent, under acidic conditions, iron compound release Fe2+ to the solution. It has been shown that the iron compound dissolves faster at lower pH than at high pH [23] . This remains in agreement with the fact, that the Fenton reaction is effective in acidic conditions. Then, Fe2+ will decompose H2O2 to generate hydroxyl radicals according to the Fenton reaction. The optimal pH in the range of (2 - 4) was reported to be a highly important factor for effective Fenton oxidation [24] -[26] . Hence, the effect of pH on colour removal estimated in between pH values varying from 2 - 4 shown in Figure 4. A decrease in the decolourization efficiency at pH 4 indicating in accordance with another report that near pH 4,
rapid H2O2 decomposition, probably on the surface of the ferric hydroxide floc, would result a fall in the appreciable amount of hydroxyl radical production [27] [28] .
Structural Elucidation of PAN
The structural elucidation and characterization of PAN were carried using NMR Spectrometer [Varian 600 MHz IFC PFG] at Inter University Instrumentation Centre [IUIC], School of Environmental Sciences, Mahatma Gandhi University. The results observed in Figure 5, the peak at 1.596 is due to the presence of water in the
![]()
Figure 4. Effect of pH on absorbance of PAN (10−-3 mol∙dm−3) irradiated 20 min by 254 nm uv-light.
![]()
Figure 5. NMR spectrum of recrystalized PAN concentration 10−4 mole∙dm−3.
solvent. Peak at 16.259 is due to impurity present. The peak at 5 is due to hydrogen bonding which may vary according to the concentration. The hydrogen present in carbon lying near to (-N=N-) is having high value 7.93 due to the presence of (-N=N-) group adjacent to it. The following data were obtained from the NMR spectrum of the recrystalized sample of PAN having concentration 10−4 mole∙dm−3.
Data obtained from Figure 5 NMR spectrum of PAN:
1) The peak at 7.93 will split into two because of presence of one neighboring Hydrogen atom.
2) The peak at 7.46 splits into three because of presence of two neighboring Hydrogen atom.
3) The peak at 5 does not split because of absence of neighboring Hydrogen atom.
4) The peak at 7.17 splits into two because of presence of one neighboring Hydrogen atom.
5) The peak at 7.82 splits into three due to presence of two neighboring Hydrogen atom.
6) The peak at 7.63 splits into two due to the presence of one neighboring Hydrogen atom.
7) The peak at 7.30 splits into three due to the presence of two neighboring atom.
The peak at 7.53 splits into two due to the presence of one neighboring atom.
Based on the above observations, the structural elucidation of phenyl azo b-naphthol (PAN), which was synthesised in our laboratory, having concentration 10−4 mole∙dm−3 as shown below.

4. Conclusions
The purpose of the study is to work out the most efficient and cost effective method for decolourization and degradation of azo dye, viz, phenyl azo b-naphthol (PAN). The present work described the use of two sources of photo dissociation such as sunlight of 495 nm and uv-light of 254 nm wavelength simultaneously
On observation of the effect of Fe2+ concentration in Fenton reagent using both sources at low pH 2 by fixing the concentration of other components in it, the decolorization efficiency increases dramatically. However, the inhibitory effect at relatively high concentration of Fe2+ ion above (3 × 10−4 mol∙dm−3) at near neutral pH 6 could be explained by an observation that Fe2+ ion are the dominant ·OH radical scavenger.
Thus, solar assisted photo irradiation and uv-light source at moderately low concentration of Fe2+ ion for performing advanced oxidation process (Fenton reaction) would be cost-effective, fulfilling energy saver management and at the same time environmental friendly too.
Acknowledgements
I acknowledge Prof. Dr. Charuvelil Aravindakumar, Dean & Hon. Director, School of Environmental Sciences, offered collaborative works at Inter University Instrumentation Centre (IUIC), Mahatma Gandhi University, Kerala, India. I would like to extend acknowledgement to my parental institution (NSS Hindu College, Changanassery, Kerala, India) and the research centre (Post Graduate and Research Department of Chemistry, SB College, Changanassery, Kerala, India).
NOTES
*Corresponding author.