1. Introduction
The development of a nuclear fuel for research reactor must take into account the non-proliferation requirements, according to the Reduced Enrichment of Research and Test Reactors (RERTR) program. To minimize a potential problem, fuels have been converted from high to low enrichment, to less than 20% of U235. However, to compensate for the resultant loss of power, fuel densities must be raised and, consequently, a new class of fuels must be developed.
Those fuels are replacing the current oxides and silicides. The most promising candidate has emerged from the binary system U-Mo, gamma phase stabilized, according firstly to the confirmation of its thermo-dynamical and irradiation behavior, observed in several “in pile” tests, performed over the last 40 years. For research reactors, γ-UMo is used in both monolithic or plate-like geometry and, in the case of dispersion, in the form of powder.
One of the techniques for the γ-UMo powder obtention is hydration-dehydration, in which hydrogen is used to promote a change in the alloy’s ductile structure into a brittle one. In the range of compositions where γ-UMo is considered as dispersed nuclear reactor fuel, 5% to 10% weight in Mo, a-U (alpha-uranium) is the proeutectoid. Thus, it is expected that the as cast and even the thermally treated structures contain some intergranular a-U, in the form of cellular precipitates, which reacts readily with hydrogen, in temperatures ranging from 50˚C to 350˚C. The formation of uranium trihydride is given by the chemical equation:
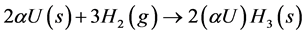 (1)
(1)
The decomposition of the γ-UMo matrix into α phase is given by:
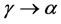 (2)
(2)
in temperatures below the eutectoid equilibrium, a total allotropic transformation, and
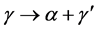 (3)
(3)
in the gamma plus alpha field, a partial allotropic transformation. Reaction (1) is called hydration (H) and its reverse, dehydration (DH). Both form the basis of the so called HDH technique. In the case of the obtention of γ-UMo powder, it is employed mainly in the range of 5 to 7 - 8%wt.
Mo.
For the powdering of metals via hydrogen, the embrittlement can be achieved primarily by a massive diffusion of hydrogen into the parent metal. Hydrogen is absorbed in the matrix lattice, in phases or precipitates, resulting in the accumulation of stresses and fracture, according to Powell [1] . For nuclear fuel alloys such γ-UMo, powdering is related also to its metastable condition, since phases of the UMo binary system behave under hydrogen in different ways. Considering the contribution of this mechanism, the transformations (2) and (3), in the γ or γ + a fields, are keys to understand how the embrittlement can be achieved via intergranular precipitation or intragranular cracking.
To produce γ-UMo powders for use as dispersed-type fuel elements in research reactors, a set of unitary operations, with or without the help of chemical reactions, has been used. The conventional ones, based on powder metallurgy such as milling, atomization, and machining, have been reported in the works of Clark et al. [2] and Wiencek and Prokofiev [3] . The HDH and HMDH (hydration-milling-dehydration) routes were studied by Balart et al. [4] , Solonin et al. [5] , Pasqualini et al. [6] , Pasqualini and López [7] .
Physical and chemical characteristics of powder and the technique of powder fabrication, influence the behavior under irradiation of the nuclear fuel particles and fuel plates. In the work of Kim et al. [8] , it was observed that atomized powders are more effective than the machined ones, in the inhibition of mechanisms of volume growth of the fuel particles. Changes in volume are mainly due to reactions with aluminum matrix, which form less dense compounds than the fuel phase. According to the rate of this undesired reaction, a volume increase of 25% was observed in mechanized powders, the double obtained by the atomized ones.
Meyer et al. [9] studied the growth of the nuclear fuel phase for atomized and machined powders in the rolling of the fuel plates. No differences were observed in the behavior of powders in γ-U10Mo, fabricated by both techniques, and no growth of the interfacial area between fuel and matrix phase was reported. However, machined powders of γ-U6Mo presented 17% in the enhancement of its total area, due to reactions with the aluminum matrix.
Hofman et al. [10] observed the formation of a phase from γ decomposition reaction, promoted by the rolling step during the fabrication of the plates, except for the atomized γ-U10Mo. The authors studied the 3 main techniques for the fabrication of γ-UMo powders and its influence in fuel behavior. A large number of bubbles were observed during the irradiation of the machined powders, probably due to their large dislocation densities, which represents sites of bubble nucleation. Also, despite the good results obtained using the atomization process, segregation, mainly at fuel densities of 8 gU/cm3 or higher, to regions outside the nucleus of the fuel plates, was also observed, due to the roundness of the particles. According to the works of Clark et al. [2] and Wiencek and Prokofiev [3] , segregation is the main undesired phenomena in the fabrication of fuel plates with the rounded geometry.
HDH or HMDH techniques are usually carried out with the help of the reaction, and by the hydrogen absorption in γ-UMo matrix, a mechanism explained by Powell [1] . The main advantage of this technique is the production of powders with intermediate characteristics between the atomized and machined powders, avoiding problems of segregation, and having nearly the same chemical behavior with the aluminum matrix such as that presented by the machined ones [3] . Thus, HDH technique can be useful for applications requiring low burnups or low fuel temperatures.
In the HDH or HMDH techniques, the usual sequence of pre-operations is the following. After casting, samples are isothermally treated in order to ensure good molybdenum distribution. The next step can be carried out in two different routes. The first is a thermal treatment in the γ + a phase field, aiming the partial conversion of the γ phase into a by means of reaction (2) and (3), followed by hydration, reaction (1), and dehydration, the inverse of the reaction (1). More recently, owing to the observation that hydrogen can be easily incorporated by γ-U7Mo [6] [7] [11] mainly in low temperatures, a previous thermal treatment is given to the samples in temperatures from 120˚C to 150˚C for times from 1 to 3 h, followed by a thermal treatment in the gamma plus alpha phase field, enabling the powdering of the UMo alloys, after the DH step. In the present work, it is analyzed the powdering of the alloys in the cooling part of the experiments, due to thermal shock, due to the observation that there is an enhancement in the powder yielding.
For both routes, it is considered that the cellular structure of the precipitates is broken in the DH step, leading to the powder formation. According to previous works [4] - [7] powder losses originated from the cellular alpha precipitates could be recovered by a new isothermal treatment, in the γ field of the UMo system. It is important to note, however, that it is only possible if the amount of molybdenum retained in the alpha phase is enough to retain the gamma metastable structure after thermal treatment.
The method presented in this paper was first devised to enable a massive powdering of the γ-UMo alloys [11] , over a broad range of Mo additions. After a series of a small scale experiments, carried out to build the curves of hydrogen absorption-desorption-reabsorption, parameters like the maximum hydrogen absorption temperatures, and temperatures of desorption or dehydration, etc., were obtained. It was observed a massive incorporation of hydrogen at the end of the experiments, mainly in the cooling ramp of the mass absorbed x time curves. The observation of the final fragmented state of the samples, together with the observations above, lead us to consider the phenomenon of thermal shock in the analysis of the experimental results.
In the experiments carried out in this study, uranium-molybdenum samples had collapsed readily, without the need of the DH step, in times and temperatures slightly lower than the reported in the literature [4] [7] . It was observed that the cooling step played an important role in the fragmentation of the samples, and one of the objectives of this work is to check this assertion. Since the same cooling rate was applied to all the samples, it is expected that those more susceptible to hydrogen embrittlement are also more susceptible to break by thermal shock. Some of the main results about the formation of γ-UMo powders, focusing the addition of 8 wt% of molybdenum, are presented and discussed.
The following analysis also leads to a new method for the production of powders and for the evaluation of an important physical parameter such as the eutectoid transformation temperature, as an alternative to the existing ones.
2. Materials and Methods
Alloys of γ-U8Mo were prepared by induction melting under high vacuum. Natural alpha-uranium cylinders and small cylindrical pieces, each having nearly 3 mm × 2 mm, of high purity molybdenum, formed the induction charge, assembled in a high purity zirconia crucible.
Samples of similar shapes were taken from the casts, in order to perform the thermal shock experiments, carried out in a thermal-gravimeter (TG) analyzer. The samples, each having nearly 200 mg, were assembled in calcined alumina crucibles, after surface polishing and cleaning. Prior to each experiment, successive operations of purge and vacuum were performed, to ensure that the internal surfaces of the equipment were free of gases and other contaminants. After a vacuum level of 2.0 × 10−2 mbar, continuous and constant flow of high purity hydrogen was inserted in the system, to the obtention of the mass-time-temperature curves, up to the end of the experiments.
Experimental inputs such as temperatures, isothermal times, heating and cooling rates, were inserted in the thermo-analyzer software, to setting up the experimental cycles. Progress of the hydrogen alloy absorption was measured as a function of time, for the given temperatures, heating and cooling rates. The strategy was to perform the experiments over a range of temperatures close to the U-Mo eutectoid transformation field. According to several authors [12] - [15] , it is in this field that the rate of γ → a decomposition is maximum. The progress of the hydrogen absorption-desorption experiments was registered as a function of time.
Powder yielding was obtained by examination of the state of the samples, after the experiments, inside the crucibles. It is a parameter, defined herein, as the relation between the total amount of powder formed and the initial sample masses, for each of the experimental conditions [11] .
3. Results and Discussions
Micrograph and X-ray spectra of the γ-U8Mo sample are shown in Figure 1; it can be observed that a high degree of homogenization is presented by the as cast samples, provided by the induction melting technique. The difference between this and the other X-ray diffraction patterns (for other additions) is represented by progressive shifts in the spectra [11] due to the presence of interstitial molybdenum in the γ-UMo body-centered cubic crystalline lattice.
Experimental results of alpha uranium hydration [11] show that rates of hydrogen absorption are higher for alpha than for gamma uranium, as shown in Figure 2, and its comparison with Figure 3. Therefore, the assessment of the initial amount of the alpha phase, due to differences in the speed of solidification and in the molybdenum concentration along the melting charge is an important parameter in the process of hydration-dehydration (absorption-desorption, in general). Furthermore, it is important to define parameters for thermal treatments steps.
Mass balance relative to the alpha phase can be expressed by the equation:
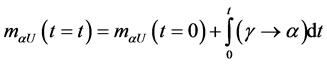 (4)
(4)
where  is the rate of gamma to alpha decomposition. Reaction 3 gives the amount of alpha phase due to the conversion of gamma. The integral, from 0 to t, gives the total amount of alpha uranium at time t due to reaction 3.
is the rate of gamma to alpha decomposition. Reaction 3 gives the amount of alpha phase due to the conversion of gamma. The integral, from 0 to t, gives the total amount of alpha uranium at time t due to reaction 3.
The γ to α term together with the hydrogen absorption by the γ-U8Mo lattice plays an important role in the mass absorption-desorption process and in the breakage of the alloys in the thermal shock phase. Therefore, the mass absorption curves reflect this behavior.
![]()
![]()
Figure 1. Left: micrograph, and right: X-ray diffraction pattern of γ-U8Mo alloy sample [11] .
![]()
Figure 2. Example of a hydrogen incorporation in an alpha uranium hydration process, γ-U8Mo [11] .
![]()
Figure 3. Experimental curves for γ-U8Mo samples [11] .
First assessment of the main mechanism of hydrogen absorption was obtained by direct comparison of the γ-U8Mo and γ-U6Mo [11] hydrogen absorption curves, the last representing an example of a massive hydration process (Figure 2). It is in accordance with curves and data from literature for this composition [4] - [7] . Explanations for this difference in behavior were given by Hoffman [10] and an experimental assessment was given by Oliveira [11] .
For γ-U8Mo alloys, the resulting hydrogen absorption-desorption curves are shown in Figure 3. The analysis is based on the temperature, which is determinant to powder formation. Also, the cooling stage of the experiments played an important role in the sample fragmentation. The mass absorption curves, including turning points of desorption-reabsorption in the cooling ramps of the γ-U8Mo alloys are also taken into consideration. Turning points are defined here as the point where desorption (or loss of hydrogen), as indicated by the decrease in mass after the isothermal treatments, is changed to a massive absorption or reabsorption of hydrogen during cooling.
The temperatures of the turning points (Ttp) were obtained experimentally from the curves. The isothermal temperatures (Tit) were the actual mean temperatures, as indicated by the thermal-analyzer. The experimental conditions, yielding results and thermal data, are shown in Table 1 and Table 2, and Figures 5-7.
Δeit and Δetp are defined as:
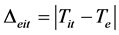 (5)
(5)
and
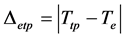 (6)
(6)
Both parameters are considered, for a first assessment of the powdering mechanism, as absolute values between the temperatures of isothermal treatments and turning points, respectively, and the theoretical (phase diagram [12] ) eutectoid transformation temperature, Te. An example of powder obtained in the experiments is given in Figure 4.
It can be noted from Table 1 and Figure 5 and Figure 6 that there is a correlation between isothermal temperatures, turning point temperatures, and the powder yielding. Since it is related with the process temperature distance from the isothermal eutectoid temperature, total hydrogen absorption and rates could also have a correlation. Results are summarized in Table 2, where the amount of hydrogen retained by the samples was calculated as a difference between the maximum hydrogen absorbed, at the end of the thermal treatments, and the amount of hydrogen released at the turning point in the cooling curves, Figure 3, Figure 4 and Figure 7. The rate at the stable hydrogen absorption is viewed as a mean value, which is calculated from the end sections of the isothermal absorption curves where the absorption is taken as constant, Figure 7.
![]()
Table 1. Yielding and thermal data from the γ-U8Mo hydrogen absorption experiments [11] .
![]()
Table 2. Hydrogen absorption data from the isothermal treatments and powder yielding, γ-U8Mo [11] .
![]()
Figure 4. SEM image of a γ-U8Mo powdered alloy [11] .
![]()
Figure 5. Δetp and powder yielding [11] .
![]()
Figure 6. Δeit and powder yielding [11] .
![]()
Figure 7. Hydrogen absorption parameters as a function of temperature [11] .
The following analysis considers two main mechanisms of hydrogen absorption for this class of alloys: the hydration reaction of the alpha phase and lattice absorption. Both lead to embrittlement and are responsible for the ease of fragmentation of the alloys, under thermal shock, such as described in the work of Powell et al. [1] and Oliveira [11] .
Differences in the densities of the γ-UMo (18.2 g/cm3), a-U (19.1 g/cm3) and a-UH3 (10.9 g/cm3) phases, can explain powdering of the alloys by hydrogen absorption, if an amount of a are present in the samples, according to Figure 2. It was predicted that hydrogen absorption in γ-U8Mo would be also given by reaction (1), and influenced by the rate at which alpha is formed. It is observed, in the work of Hoffman et al. [10] and from the U-Mo TTT (time-temperature-transformation) diagrams [12] - [15] , that the rate at which gamma decomposes to alpha in an homogeneous alloy is dependent on the isothermal treatment temperature, having a typical C shape profile. The decomposition is more pronounced when the system is close to the eutectoid temperature.
Thus, the ease of the hydrogen absorption-desorption process in the embrittlement of γ-UMo alloys and the fragmentation leading to powdering must be, at first, influenced by the total amount of α-U phase. It continuously changes by the term related to the cellular matrix decomposition reaction, the integrand in Equation (4), which leads to its precipitation in the grain boundaries and migration out of the gamma matrix. Thus, the highest the molybdenum content, the lowest is the sample fragmentation, by means of the reaction (1).
The rate of the nucleation of a phase in a γ UMo matrix can be given by the Wolmer-Weber [16] expression:
![]() (7)
(7)
where Nj/V is the number of nucleation sites with size j per unity volume, Aj is the frequency factor, Ui is the activation energy for the diffusion of atoms through the interface of the nuclei, Uj* is the activation energy for the formation of critical nuclei of size j. R is the universal gas constant and T the absolute temperature.
After some simplifications, an expression for the time for nucleation ti with temperature can be obtained:
![]() (8)
(8)
enabling the construction of the TTT UMo diagrams. Here, in a curve of lnti x (1/T), its slope gives the activation energy for interfacial diffusion. Theoretical graphs based on (08), for molybdenum additions of 5 to 12 wt%, are given in the work of Hofman et al. [17] , showing that the addition of Mo increases the energy for the formation of alpha nuclei. Thus, there is an increase in the stability of the UMo alloys, leading to a lower rate of hydrogen absorption.
Similar curves, but based on empirical data, are given in the works of Van Thyne and McPherson [12] [13] and McGeary [14] , where the inflexion or nose points of the TTT diagrams changes, according to the methods employed for the determination of the gamma-alpha equilibrium. From McGeary [14] , maximum decomposition of γ-U8Mo occurs at 525˚C, as determined by X-ray diffraction and metallographic techniques. According to Van Thyne and McPherson [12] [13] , resistivity methods indicated a value of 500˚C, and hardness methods, 570˚C. Since the present study deals with powder fragmentation, it is reasonable to suppose that the value where there is a change in hardness for the γ-U8Mo is the one where there was a higher concentration of alpha phase, leading to a higher increase in the total hydrogen absorption via hydration and to more powder formation. According to Figure 5 and Figure 6, this occurs at a value near 568.4˚C or 573.5˚C, where the highest values in the hydrogen absorption and absorption rates were obtained; but the yielding in fragmentation was only 12%.
The γ + a → γ transition temperature in the U-Mo phase diagram for γ-U8Mo is approximately 619˚C, which is far from the eutectoidal transformation temperature and closer to this transition line, when the treatment is applied at 570˚C. Thus, the inflexion point, i.e., the point of the maximum decomposition, is reached at 568.4˚C, instead of 573.5˚C. The corresponding turning point temperature is then at a minimum distance from the classical eutectoidal transformation temperature, and can be related to a minimum in the energy, Equation (7), to break up the samples by the thermal shock technique.
Considering the same cooling rate in all the experiments, it is possible to conclude, from Figure 5 and Figure 6, that yielding was better with the smallest temperature difference, leading us to setup the temperature of 568.4˚C as the closest to the real eutectoidal transformation temperature.
Absorption in the lattice is the mechanism of hydrogen incorporation related to the presence of the interstitial positions. Together with the inter-granular precipitation phenomenon analyzed above, both are responsible for the mechanical instability of the alloys, under thermal shock. Thus, it is predicted here that if the distance from the eutectoid line increases, the system is far from the condition that leads to more alpha precipitation, and thus an absorption mechanism related to the interstitial positions, also called embrittlement by hydrogen saturation and tension sources creation [1] , is the one which explains the embrittlement of the alloy and the good powder yielding. This phenomenon can also explain absorption in temperatures higher than the eutectoidal resulting, however, in low yielding. Hydrogen was released due to desorption, due to its poor incorporation in these samples. By means of Figure 3, Figure 5 and Figure 6, this mechanism can be easily visualized.
According to the phenomena described above, some of the physical characteristics of the powders can be inferred by the huge increase in the absorption rates, mainly in the cooling part of the curves. In all experiments in which good yielding was obtained, it was observed that, at some point in the cooling ramp, there was a change in the mechanism of mass absorption. Considering that absorption is a phenomenon dependent on the amount of free area existent in the system, it is expected that the higher the surface of the material exposed to the gas, the higher is the absorption, making it possible to estimate the dimensional parameters of powders, such as mean diameters.
The change of the absorption rate that follows a brief period of mass loss can be explained by the following hypothesis. Hydrogen diffuses through the samples at a determined rate, and reacts with alpha uranium as it is precipitated in grain boundaries during the isothermal treatment. With the progress of the reaction, absorption increases, also increasing absorption by the gamma uranium-molybdenum lattice. At some specific point where the isothermal treatment ends and the cooling ramp starts, the differences in the crystalline structure of the parent phase and uranium tri-hydride leading to differences in thermal expansion or contraction, cause the sample to collapse. This collapse leads to an increase in the absorption rate and thus to the observed powder formation. Therefore, it is expected, as it was observed, that absorption increases with time in the cooling ramp region.
Considering the level of hydrogen exposition, assessed by means of Figure 7, it is important to observe that, owing to the low solubility of hydrogen in uranium and uranium alloys, and to the strong tendency to form hydrides, the main mechanism of embrittlement of uranium alloys must be the stresses induced by alpha uranium hydride formation, mainly in the low stabilized alloys of γ-UxMo (x < 8).
Equations (1) to (7) are the basics for the understanding of the behavior of the alloy in terms of equilibrium and production of potential sites or hydrogen absorption. However, some other phenomena must occur in order for hydrogen to be absorbed in the alloys.
Since the environment of the furnace is filled with gas, diffusion of hydrogen through the samples must be analyzed. For a concentration profile to be built, c(x, t), it is necessary to know its concentration over the surface of the samples, cs. Diffusion equation leads to:
![]() (9)
(9)
where D is the diffusion coefficient of hydrogen in the γU lattice, having a strong temperature dependency. It can explain the low powder yielding for temperatures below the eutectoidal. In this particular case, both diffusion and γ decomposition occurs at low speed.
For the diffusion through the surface of the samples, at the beginning of the experiments, co = 0, no hydrogen is assumed to be present in the material’s surface. Hydrogen concentration at the samples’ surface, cs, can be estimated considering that it is proportional to the surface area of the samples. However, only a fraction of the sites in this surface must be available for adsorption. In terms of numbers of atomic sites in the surface of the metal, NHm, and the interstitial sited for hydrogen absorption, NHs, a fraction of sites available cHs can be represented by:
![]() (10)
(10)
for atoms on the surface. An estimative of cs related to the density of the UxMo alloy, rx, and to the thickness a of the metal’s monocrystalline surface shell, can be given according to [11] :
![]() (11)
(11)
where the parameter a is obtained from the most intense experimental peaks of the X-ray spectra of the alloy and its density, both functions of the amount of Mo in the alloy. Results are given in the Table 3.
From the phenomenology described here, which leads to the prediction of the behavior of the alloys under thermal shock, it can be derived estimative of some of their mechanical properties. Since the analysis is based on the reactions (1) to (3), the properties of the aU, γ-UMo and a-UH3, can be inferred. The objective is to determine how volume changes during the decomposition reaction γ → a, and also during the hydride formation, leads to a reduction in the tensile strength of the alloys. Due to the differences in density, it is expected that production of alpha and absorption of hydrogen causes a substantial change in volume, which causes the breakage of the alloy under the heating and the cooling parts of the thermal treatment curves.
Based on the correlations from the work of Waldron et al. [18] some mechanical properties of interest can also be estimated. Measurements of γ-U8Mo hardness [11] - [13] enabled the estimates of some of its mechanical properties, such as given in Table 4, where E is the elastic modulus and Y the yield strengths at the indicated temperatures (˚C).
Having the elongation of the alloy in terms of temperature, it is possible to estimate the times for the sample’s breakage, based on some expression of the thermal expansion coefficient, λ, for this alloy. Data in literature for γUMo alloys can be found in the works of Saller et al. [19] and Repas et al. [20] , for the additions of 8, 9 and 10 wt% Mo. Following Repas et al. [20] , γ-U8Mo, the values of λ are 18.8 × 10−6/˚C from 500˚C to 575˚C, and 20.7 × 10−6/˚C, in the γ region. From the work of Blackledge and Libowitz [21] the linear thermal expansion coefficient of uranium trihydride is estimated as 1.631 × 10−5/˚C. Thus, the evolution of the alpha phase presented could be determined, and also the hydrogen consumption by hydration. Changes in the matrix volume can also be determined and, thus, the time for the structure to collapse, during heating and/or cooling.
According to our hypothesis for the transformation reaction γ → a, it can be verified a similarity with the C
![]()
Table 3. Surface concentration of H, cs, for samples of γ-U8Mo [11] .
![]()
Table 4. Estimated mechanical properties of γ-U8Mo alloy [11] .
shape curves from the TTT diagrams (in our case, due to the inversion of the axis, it resembles a “U” in Figure 5). Thus, it is inferred that absorption of hydrogen will follow the same profile. Also, if the amount of alpha phase is greater as it approaches the maximum temperature of decomposition, more easily the embrittlement is reached, by the inter-granular precipitation mechanism, as shown from the results above. This phenomenon lowers the thermal shock resistance of the alloys, leading to the alloys rupture in the chosen experimental temperatures, and explaining the C (or U) shape of the curves of ΔTe shown in this work.
4. Conclusions
The conversion of the γ as cast structure into a-U is thoroughly mentioned in the literature as a pre-requisite to the obtention of good powder yielding. It was experimentally demonstrated here that, using a convenient set of parameters, fragmentation of the structure by thermal shock could be obtained even for 8 wt% Mo alloys (instead of 6 to 7 wt% Mo as referred in literature), but in more restrictive conditions of temperature, near the eutectoid isotherm. In addition, isothermal treatments in temperatures far from the eutectoid were also applied to some samples, also leading to the possibility of the obtention of some γ powdering, but with low yielding.
In terms of the fabrication process, better yielding could be achieved applying only thermal shock, on samples under a thermal treatment carried out in temperatures of 568.4˚C. It was observed that the greater the a content of the samples is, the greater the yielding is. With the help of hydrogen, and with the observation that the maximum powdering of the alloys occurred near to the eutectoid temperature transformation, a value of 568.4˚C was suggested here for this isotherm in the U-Mo system. Therefore, hydration of the alloys could also be used as a method to determine some important values of equilibrium properties in the uranium-molybdenum system.
During the powdering of the samples, a number of sites are created by the particles detached from the initial samples, which are capable of absorbing more hydrogen, until its saturation point. The creation of new absorption sites can be related to the difference between the total amount of hydrogen absorbed at the maximum in the isothermal treatment and the minimum in the cooling ramp, after which the system undergoes a new increase in the absorption rates. This increase allows us to estimate the surface area created and the mean particle diameter, produced in each experiment. Thus, powdering of the alloys can be achieved without mechanical means, in the hydration phase of the process, provided a good speed of cooling being given to the samples. A reduction in the number of steps of the traditional HDH or HMDH can be obtained.
On the basis of the classical time-temperature-transformation diagrams for the binary uranium-molybdenum system, and based on the results of stability under aluminum and oxygen [11] [22] , it can be suggested that compositions near or between γ-U7.5Mo and γ-U8Mo are the most gamma stable, in terms of reactions with the aluminum matrix. Since their behaviors under irradiation, according to previous works [8] -[10] [23] are almost the same in the range of 5% to 8% Mo addition. The compositions above could be useful as a fuel phase in nuclear research reactors, mainly to its stability with aluminum, an advantage in the fabrication of the fuel plates.
Same kind of work is done for other compositions, and a publication is being prepared, concerning composition and a global analysis of all the γ-UMo alloys studied.