Preparation and Characterization of MMT Doped PVA/SA Polymer Composites ()
1. Introduction
Polymer blending is one of the most important contemporary ways for the development of new polymeric materials. It is a useful technique for designing materials with a wide variety of properties [1] . The structural, optical, thermal and electrical properties of blends can be suitably modified by the addition of dopant depending on their reactivity with the host matrix [2] . It enfolds the advantage of both polymer and filler components leading to a wide spectrum of applications [3] - [5] . Nano composites have a significant role in tailoring the properties of polymeric materials to suit any technology [6] [7] . PVA is a water soluble polymer which is in widespread use in several interesting physical properties and very useful in technical applications in biochemical and medical. PVA is a potential material having a good charge storage capacity and dopant-dependent electrical and optical properties [8] [9] . PVA has the different internal structure which may be considered as amorphous or semi crystalline. The semi crystalline structure of PVA shows an important feature rather than the amorphous one [10] . Polyvinyl alcohol (PVA) is a transparent, water-soluble and biodegradable polymer with many technological, pharmaceutical and biomedical applications [11] - [13] . Sodium alginate (SA), a biodegradable polymer, is a negatively charged polysaccharide derived from brown sea weed [14] . The unique properties of sodium alginate are its biological origin, non-toxicity, hydrophilicity, biocompatibility and low cost [15] [16] . PVA interacts with SA through hydrogen bonds to form PVA/SA composite films [17] . Important aspects of these PVA/SA films are quite smooth, uniform, flexible, and transparent.
In this work we have doped Montmorillonite (MMT) to PVA/SA polymer blend and characterized the prepared films using various techniques. The obtained results have been quantified in terms of microstructural parameters.
2. Materials and Methods
2.1. Materials
Montmorillonite (MMT) belongs to soft phyllosilicate group of minerals. Chemically it is Hydrated sodium calcium aluminium magnesium silicate hydroxide (Na,Ca)0.33(Al,Mg)2(Si4O10)(OH)2nH2O. Polyvinyl alcohol (PVA)-[CH2CH(OH)]n is a semi crystalline material. It has dopant-dependent optical, electrical and thermal properties. Sodium alginate (SA)-[C6H7NaO6]n is a non-toxic, hydrophilic, biocompatible, biodegradable and low cost material.
2.2. Preparation of MMT Doped PVA/SA Films
Samples were prepared using solution casting method. 5% PVA solution and 1.5% SA solution was prepared by dissolving the requisite quantity in 500 ml distilled water using a magnetic stirrer. Different concentrations of PVA/SA solutions (10/90 to 90/10) were mixed and stirred for 2 hours. Later the solution is casted on plastic petri dishes and allowed to dry completely at room temperature. After complete drying, the films were peeled out. The film with 70/30 concentration was found to be blended well, hence the solution of this percentage is used to prepare the different weight concentrations of MMT doped PVA/SA films and used for further studies.
3. Experimental
3.1. X-Ray Diffraction Studies
XRD patterns of pure PVA/SA and MMT doped PVA/SA films of different concentrations were recorded using Rigaku Miniflex II Desktop X-ray Diffractometer equipped with CuKα radiation (wavelength = 1.5406 Å) and a graphite monochromator. The samples were scanned in the 2θ range 6˚ - 60˚ and the specifications used for the recording are 30 kV and 15 mA with 5˚/min. The recorded XRD patterns of the films are shown in Figure 1.
![]()
Figure 1. X-ray diffraction patterns of MMT doped PVA/SA films.
3.2. Impedance Measurements
The impedance and phase angle of these polymer samples were measured in the frequency range between 5 Hz - 500 kHz using Electrochemical Impedance Spectroscopy (ZAHNER) IM6.
3.3. Ultraviolet and Visible Spectroscopic Studies
The variation of transmittance (T) as a function of wavelength (λ) for PVA/SA and PVA/SA: MMT (0.1% - 0.4%) was recorded at room temperature using Labtronics MODEL LT-2800 double beam UV-visible spectrophotometer. The recorded UV-visible spectra of the MMT doped PVA/SA films patterns of the films are as shown in Figure 2.
3.4. FTIR Studies
The Infrared transmission spectra of these polymer samples were recorded at room temperature in the range of 4000 - 500 cm−1 using Perkin Elmer Spectrum. The recorded FTIR spectra of pure and MMT doped PVA/SA films are given in Figure 3.
4. Results and Discussion
4.1. X-Ray Diffraction Studies
X-ray diffraction patterns do indicate the presence of crystalline and amorphous regions. The total strain in crystal is due to lattice mismatch between the material and the substrate and other crystallographic defects that may be present in the crystal. If the size and strain broadening are present simultaneously then crystallite size and strain may be obtained from Williamson-Hall plot [18] [19] . The slope of the plot represents the average strain in the crystal whereas the intercept gives the crystalline size according to the relation.
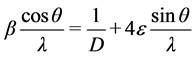 (1)
(1)
![]()
Figure 2. UV-visible spectra of MMT doped PVA/SA films.
![]()
Figure 3. FTIR spectra of pure and MMT doped PVA/SA films.
where β is Full Width at Half Maxima (FWHM), D is the average crystallite size and ε is average strain. Plot of
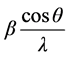 verses
verses 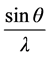 gives a straight line with y intercept equal to the inverse of the size and the slope equal to the micro strain present in a given sample.
gives a straight line with y intercept equal to the inverse of the size and the slope equal to the micro strain present in a given sample.
This approximated method makes it possible to obtain a qualitative mean value characterizing each of the effects that cause the increase in peak width. If the slope of the line is almost horizontal, then the sample contains only a small amount of micro strains. On an average, we observed that the crystallite size of pure polymer blend to be decreased with the increase in dopant concentration. This is because of the nanoparticles which make the polymer blend more amorphous with concentration. Since the added concentration of the nanoparticle is very small it does not show any prominent reflections in the XRD patterns but it effects internally in the broadening of obtained reflection for pure polymer blend and thus results in the decrease of overall crystallite size of the parent polymer matrix. Microstructural parameters of these pure PVA/SA and PVA/SA: MMT composites MMT are given in the Table 1.
4.2. Conductivity Measurements
Prepared films of known thickness “d” were placed between the electrodes of known area “A” of a.c. impedance spectrometer. The bulk resistance Rb of the film is calculated using the relation 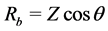 [20] , where Z is impedance and θ is the phase angle. Ionic conductivity “S” of the polymer sample is calculated using the relation.
[20] , where Z is impedance and θ is the phase angle. Ionic conductivity “S” of the polymer sample is calculated using the relation.
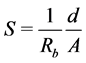 (2)
(2)
Impedance, bulk resistance and conductivity of pure PVA/SA and PVA/SA films doped with different concentrations of MMT are given in the Table 2. From Table 2, we observed that the conductivity increases with
![]()
Table 1. Microstructural parameters of MMT doped PVA/SA films.
![]()
Table 2. Impendance, bulk resistance and conductivity of the MMT doped PVA/SA films.
increase in the concentrations of MMT. A least squares plot of 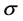 vs
vs 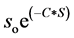 gives a value of S as 9.6%. Here S represents fractional change in interfacial surface area value with the surrounding polymer network. There is a net increase of 9.6% of interfacial surface area of MMT with PVA/SA network. This is significant which indicates the changes in the physical as well as environmental properties of the composite.
gives a value of S as 9.6%. Here S represents fractional change in interfacial surface area value with the surrounding polymer network. There is a net increase of 9.6% of interfacial surface area of MMT with PVA/SA network. This is significant which indicates the changes in the physical as well as environmental properties of the composite.
4.3. Ultraviolet and Visible Spectroscopic Studies
A casual glance at Figure 2 indicates that PVA/SA has high transmission and it decreases with the increase in concentration of MMT in doped films. Further, it is interesting to note that these films have absorption at wavelength which corresponds to the energy of 1 eV which indicates in a way that these films do behave as if there is an energy gap similar to those in semiconductors. This value decreases with increase in concentration of MMT. This is due to the formation of intermolecular hydrogen bonding between the ions of the dopant and the OH groups. The decrease in transmission for doped PVA/SA films reflects the variation in the optical band gap which arises due to the change in polymer structure. Also it is found that as the concentration of MMT increases the transmittance edge shifts towards the lower wavelength region (773 nm to 769 nm).
4.4. FT-IR Studies
The spectra exhibit bands characteristic of stretching and bending vibrations of the films. FT-IR absorption bands positions and the assignments of all the prepared samples are listed in Table 3. From the spectra, for pure PVA/SA the broad band at about 3309 cm−1 is assigned to O-H stretching vibration of hydroxyl group. SA is anionic polysaccharide with a carboxylate group in the side chain. This makes it a good biocompatible and biodegradable. Hydrogen bonding is formed between carboxyl group of SA and hydroxyl group of PVA. The band corresponding to CH asymmetric stretching vibration occurs at about 2914 cm−1. The band at about 1086 cm−1 corresponds to C-O stretching of acetyl groups present on the PVA backbone. The vibrational band at about 1734 cm−1 corresponds to C=O stretching of PVA and SA [21] [22] . The band at about 836 cm−1 is attributed to AlMgOH bending vibrations [23] . In the case of MMT filled PVA/SA, FR-IR spectra shows shifts in some bands (1423 cm−1, 1243 cm−1 and 1086 cm−1) which is essentially due to Si-O stretching: out of plane and in-plane which is absent in pure films. Some shift is observed here in these films due to constraints of neighbouring polymer network. 1423 cm−1 is normally assigned to carboxyl group. This indicates the considerable interaction between the polymer and dopant. Addition of 4% of MMA, Si-O-Si with anti symmetric stretching mode is visible around 1100 cm−1. In pure PVA/SA, the intensity is around 90, and then it changes to 85 (0.1% of MMT), less than 85 (0.2% of MMT), less than 80 (0.3% of MMT) and less than 70 (0.4% of MMT). Obviously this stretching has been damped by the interface surrounding MMT in polymer network.
![]()
Table 3. Assignment of IR characterising peaks for prepared MMT doped PVA/SA films.
5. Conclusion
Preparation and characterization of PVA/SA films have shown interesting results like conductivity, transparency and energy gap. The increase in concentration of MMT nanoparticles, increases the conductivity of nanocomposite by decreasing the energy gap. This is in conformity with the variation of microstructural parameters computed from X-ray line profile analysis. Reason for such a change in the parameters of these polymers is due to the formation of hydrogen bonding between PVA and SA. In fact such bonding alters the polymer network and hence crystallite size. It is important to note that these changes are due to fractional change in interfacial surface area of about 9.6% which has been computed using conductivity data. Further, FTIR analysis shows that the band at about 836 cm−1 is attributed to AlMgOH bending vibrations. In the case of MMT filled PVA/SA, FT-IR spectra show that shifts in some bands (1423 cm−1, 1243 cm−1 and 1086 cm−1) are essentially due to Si-O stretching, which plays a significant role in the final property of the composite.
Acknowledgements
Mrs K.H.L thanks UGC for providing fellowship for this research under Faculty Improvement Program. Authors thank UGC, India for grants in the form of UPE/CPEPA projects. Thejas Urs G and Dr Mahadevaiah’s help in preparing the samples and manuscript is acknowledged.
NOTES
*Corresponding author.