A Phase II Study of Antineoplastons A10 and AS2-1 in Adult Patients with Recurrent Glioblastoma Multiforme: Final Report (Protocol BT-21) ()
1. Introduction
Glioblastoma multiforme (GBM) is the most common highly-malignant brain tumor with an average annual incidence in the U.S. of over 50,000 cases [1] . It is estimated that over 90% of cases of this patient population will develop recurrence of the disease (RGBM). It is currently thought that GBM develops through the progressive accumulation of epigenetic and genetic changes that help the cells to escape destruction by molecular mechanisms of the body [2] . Hereditary factors seem to contribute to less than 1% of GBMs [3] . On the other hand, there is vast literature indicating that environmental exposure is associated with increased incidence of GBM. Ionizing radiation is the only environmental factor that is consistently associated with the development of GBM [2] [4] . Chemical exposure as a possible cause of glioma is an actively debated issue for over 30 years [5] . A number of chemicals have been identified that induce brain tumors in animals, but the evidence from the epidemiological studies are not conclusive [2] [6] -[8] . The interesting results were found in epidemiologic studies of association between allergies and cancer [9] . Allergic condition appeared to increase the risk of various types of cancer, but there is documented inverse relationship between allergies, asthma, and immunoglobulin E levels and risk and prognosis of glioma [10] . GBM has inherent feature to progress due to accumulation of epigenetic and genetic abnormalities over time, which is similar to other aggressive types of cancer [11] . The main difference between these cancers and GBM is an extremely complex network of abnormal genes in GBM [12] . Unstable GBM genome undergoes continuous changes and converts to RGBM after standard-of-care treatment [13] [14] .
Standard of care treatment for newly-diagnosed GBM consists of surgical resection followed by radiotherapy (RT) with concurrent temozolomide (TMZ) and six maintenance cycles of adjuvant TMZ [15] . The improvement in overall survival in such treatment is mostly restricted to a subgroup of patients with silenced DNA repair gene 0-6-methylguanine-DNA methyltransferase (MGMT) [16] . MGMT promoter methylation seems to be associated with improvement of progression-free survival (PFS) (8.7 vs. 5.7 months) and overall survival (OS) (21.2 vs. 14 months) [17] . Unfortunately, over 90% of patients suffer recurrence [18] . A small number of patients with recurrent glioblastoma multiforme (RGBM) may undergo a second surgery or RT. In patients who relapsed after TMZ, 20% PFS at 6 months can be accomplished with nitrosoureas, TMZ in different dosing regimens or bevacizumab [19] [20] . It is a general consensus that more research is needed to introduce better treatment regimens for patients with RGBM [19] .
ANPs are synthetic derivatives of glutamine, isoglutamine, and phenylacetic acid [21] . The initial studies revealed objective responses and long-term OS in patients diagnosed with GBM [22] . With positive preliminary data, we have designed and conducted a single-arm Phase II study of ANP A10 and AS2-1 in patients with recurrent high-grade glioma with special emphasis on RGBM.
2. Patients and Methods
2.1. Patient Population
The inclusion criteria of the protocol required the age of the patients to be over 18 years, a resected or biopsied tumor prior to RT, and radiologic evidence of recurrent tumor by gadolinium-enhanced magnetic resonance imaging (MRI) performed within 14 days before initiating the study. It was requested that there should be a complete recovery and at least four weeks from prior surgical procedure. The Karnofsky Performance Status (KPS) should be between 60 and 100, hemoglobin concentration of at least 10 g/dl, a white blood count of at least 1500/mm3, and a platelet count of at least 50,000/mm3. The subjects’ total bilirubin and serum creatinine concentrations should be no higher than 2.5 mg/dL, and aspartate aminotransferase (AST) and alanine aminotransferase (ALT) no higher than five times the upper limits of normal. The exclusion criteria did not permit admission of pregnant or breast-feeding females, patients with high medical or psychiatric risks due to non-malignant disease, presence of active infection, chronic heart failure, serious lung disease, uncontrolled hypertension and inadequate hepatic function, brainstem location of the tumor and patients incompetent to give informed consent for treatment. The use of corticosteroids was permitted to reduce symptoms and signs attributed to cerebral edema, but it was recommended that the smallest doses compatible with the preservation of optimal neurologic function, be used. Confirmation of the pathologic diagnosis by an outside pathologist was also required. Patients were removed from the study if 1) progressive disease (PD) developed, 2) if toxicity levels became unacceptable, 3) if the subject developed a concurrent illness that interfered with therapy, 4) if the subject or guardian asked to be removed from the study or became non-compliant with the study criteria, and 5) if the subject completed at least 8 months of treatment after determination of complete response (CR), partial response (PR) or stable disease (SD). All study subjects and/or guardians read, understood, and signed written informed consent prior to enrollment. This study was conducted in accordance with the U.S. Code of Federal Regulations, Title 21, Parts 11, 50, 56, and 312; the Declaration of Helsinki (1964) including all amendments and revisions; the Good Clinical Practices: Consolidated Guideline (E6); International Conference on Harmonization; and the FDA’s Guidance for Industry. The study was sponsored by the Burzynski Research Institute, Inc., (BRI) and conducted by the Burzynski Clinic (BC) in Houston, Texas. The patients did not pay for the investigational agents.
2.2. Study Design
The study was designed as a single-arm, two-stage, interventional Phase II trial of ANP A10 and AS2-1 as the single therapy modality in a high-risk, poor-prognosis study population [23] . The study was listed by the National Cancer Institute (NCI). It was supervised by the independent Institutional Review Board (BRI-IRB, BCBT-21). The study was performed according to Protocol BT-21 which was submitted to the FDA under the IND 43,742. The study commenced on February 26, 1996, and closed to accrual on February 25, 2011. The protocol was amended by BRI several times; however, none of the amendments altered the aim or design of the original study objectives/outcomes.
3. Statistical Consideration
The sample size and statistical methods were based upon the method described by Chang et al. and were described before [24] . A response rate to ANP of ≥10% was considered “of interest”, and the primary endpoint was to determine the overall response rate (OR = CR + PR) to ANP therapy. An interim analysis would be conducted after 20 subjects had enrolled in the study. If 1 or more patients achieved a confirmed radiographic response, an additional 20 subjects would be recruited. OS was measured from the first day of ANP administration until death from any cause, and progression-free survival (PFS) was measured from the first day of the treatment until the date of first observation of PD, beginning of other treatment or death. OS and PFS were estimated by Kaplan-Meier analysis using the MedCalc Statistical Software version 13.3 (MedCalc Software bvba, Ostend, Belgium; 2014. The primary endpoint was the response to treatment which was determined in these different populations: 1) intent-to-treat (ITT) population, 2) RGBM, and 3) RGBM patients who received at least 28 days of ANP (ERGBM). Time to best response, dosage, and duration of treatment were analyzed. The average maximum effective daily dose and the range were calculated.
4. Treatment Plan
ANP A10 and AS2-1 were the only anti-tumor treatments administered in this study. The formulations were delivered via a dual-channel infusion pump and single-lumen subclavian catheter (Broviac or Groshong) every 4 hours. A loading dose of 72 g of A10 in a fluid formulation of concentration 0.3 gram/mL and 16 g of AS2-1 in a fluid formulation of 0.08 mL was distributed evenly into 6 single doses after an initial testing dose of 10 mL of each. The next day the dose was increased by 72 g and 16 g respectively and the escalation was repeated daily until maximum tolerated dose was established. When the study subject reached the highest tolerated dose, but not higher than 20 g/kg/day of A10 and 0.4 g/kg/day of AS2-1 the “escalation phase” of the treatment stopped and the subject continued the treatment which required daily administration of 6 doses of A10 and AS2-1 (every 4 hours). The study subject continued the established dose until a response to the treatment was determined, and it was advised to continue treatment for at least eight months after CR was documented. Dosing was stopped at patient request or if their clinical condition worsened. After discontinuation of IV ANP, the patients were eligible for a maintenance treatment with 0.5 g capsules of A10 and AS2-1. According to the maintenance plan, patients were taking up to 2.5 g six times a day of both A10 and AS2-1. The escalation of the dosage of ANP was required based on the results of prior studies [21] to find out if the patients were able to tolerate large volume infusions of intravenous fluids associated with higher doses of ANP. As a safety precaution it was recommended that the escalation of the dosages will continue through Phase II and Phase III trials programs. Medications that were considered necessary for the subjects’ welfare and that did not interfere with the evaluation of treatment were given at the discretion of the investigator. The use of corticosteroids was carefully monitored. Subjects received full supportive care, including transfusions of blood products and antibiotics when appropriate. No other anticancer mediation was permitted.
The initial two weeks of therapy was administered by BC staff on an outpatient basis. Subjects and/or their legal guardians were trained by radiological clinic staff to self-administer ANP therapy. Based on prior experience, starting on week 3 ANP therapy was administered by the enrollee or their guardian at home. Treatment and monitoring of the subject’s condition, once released to self-administered therapy, continued under the supervision of the subject’s local physician.
5. Evaluation and Follow-Up
Prior to the start of treatment, a gadolinium-enhanced magnetic resonance imaging (MRI) measured all contrast-enhancing lesions. The products of the two largest perpendicular diameters (LPD) of all lesions were calculated and totaled, providing a baseline evaluation for each study subject. As a common practice at that time in other clinical trials, the tumor measurements were based on contrast enhanced lesions, but the overall tumor size was also measured including T2 and FLAIR images [19] [23] . The baseline provided the reference for determining response outcomes to the treatment. Blood and urine tests (complete blood count with differential, platelet count, reticulocyte count, and serum chemistry) anticonvulsant serum levels, prothrombin time, and partial thromboplastin time were carried out on all subjects prior to the start of treatment to establish normal baselines. The additional pretreatment measurements included KPS, vital signs, clinical disease status, demographics, medical history and current medications, physical examination with neurologic emphasis, chest X-rays and EKG. Toxicity was evaluated in ITT population. Data on adverse experiences (AEs) were collected during the initial 3 weeks of ANP therapy by clinic staff at subject request. In accordance with other Phase II studies conducted at the initiation of this trial, the possible responses to the treatment were CR, PR, SD and PD. CR required the disappearance of all enhancing lesions, sustained for at least 4 weeks, and only physiologic replacement doses of steroids were acceptable. PR required 50% or higher decrease of the LPD of enhancing lesions and stable or reduced corticosteroid doses. PD was determined when there was over 50% increase of enhancing lesions or new lesions, and SD was the status between PR and PD. The results of all MRI and PET scans were verified by radiologists not affiliated with BRI or BC and their determination of response was accepted. Study subjects were categorized by their overall best response during the course of the treatment. The duration of each response was measured from the date that the criteria for the outcome were first met until the date that PD was first documented. The original protocol required more than 50% increase of tumor size for PD, but in this publication 25% increase was acceptable and all cases reported in this paper were re-evaluated by radiological RANO criteria to allow comparison with other studies. In the case of SD, the duration was measured from the time therapy commenced. During the study the generally accepted criteria for evaluation of responses in high-grade glioma changed toward reliance on OS and PFS rather than tumor responses. As a result, the protocol was amended to include also survival analysis. Correlative studies including 1p19q deletions and MGMT silencing were not performed because they were not yet introduced when the patients were accrued except for the three patients who were accrued before completion of the study.
6. Results
6.1. Patient Demographic
Forty candidates were registered in the study (ITT population). There were 35 cases of GBM, 4 cases of anaplastic astrocytoma (AA) and 1 case of anaplastic mixed glioma (AMG). The characteristics of the patients are listed in Table1
Among 35 patients diagnosed with GBM, there were 30 cases of RGBM and 5 cases of persistent GBM. In the group of 30 RGBM patients, there were 24 subjects who received at least 28 days of ANP (ERGBM). The characteristics of GBM patients are presented in Table2
Among three patients who had genomic analyses, none of them had IDH1 mutation. One of these patients could be assigned to the subgroup of classical GBM [12] . He had amplified EGFR and silenced MGMT and developed PD during the treatment with ANP. Two remaining patients had reduced, but active MGMT and one of them could be assigned to a proneural subgroup. Both of them obtained stabilization of disease.
6.2. Treatment
In the ITT group the median daily dose of ANP A10 ranged from 1.27 to 11.88 g/kg/d with a median of 6.52 g/kg/d. For AS2-1, the median daily dose was 0.23 g/kg/d, with a range of 0.11 to 0.38 g/kg/d. The median daily

Table 1 . Study population demographics—study BT-21.
AA—anaplastic astrocytoma; AMG—anaplastic mixed glioma; GBM—glioblastoma multiforme; RT—radiation therapy; CH—chemotherapy.

Table 2. Characteristics of glioblastoma multiforme patients—study BT-21.
ERGBM—eligible recurrent glioblastoma multiforme; GBM—glioblastoma multiforme; RGBM—recurrent glioblastoma multiforme.
dose of A10 in subjects until first OR was 4.97 g/kg/d; (range 4.59 to 6.99 g/kg/d) and the median daily dose of AS2-1 was 0.26 g/kg/d; (range 0.22 to 0.28 g/kg/d). The median time to first OR was 46.5 weeks (range 37.1 to 58.6 weeks). In the group of subjects with an SD response, the median daily dose of A10 was 7.14 g/kg/d (range 3.67 to 9.59 g/kg/d) and the median daily dose of AS2-1 was 0.26 g/kg/d (range 0.22 to 0.38 g/kg/d). The duration of IV ANP therapy ranged from 0.4 to 58.6 weeks with a median of 11.9 weeks. One PR patient from RGBM group was eligible for a maintenance oral treatment of A10 and AS2-1 capsules. One additional SD patient from the persistent GBM group was given maintenance oral treatment. In the RGBM group, the median daily dose of ANP A10 ranged from 1.27 to 11.88 g/kg/d with a median of 6.95 g/kg/d. For AS2-1, the median daily dose was 0.24 g/kg/d, with a range of 0.15 to 0.38 g/kg/d. The median daily dose of A10 and AS2-1 for OR is the same for ITT and RGBM. The median duration to the first OR was 10.9 weeks (range from 0.43 to 58.3 weeks). One SD RGBM patient received a median dose of A10 9.74 g/kg/d and AS2-1 0.26 g/kg/d. There was no statistical difference from the ERGBM group and the RGBM group when comparing the median daily doses and time to first OR, since ERGBM is a subgroup of RGBM.
7. Responses and Survival
The responses were evaluated separately for all patients (ITT), for the group of RGBM and for ERGBM. Four patients obtained OR (10%, 13.3% and 16.7% for ITT, RGBM and ERGBM respectively). The responses of two of them were classified as CR, and the other two as PR (Figure 1).
Four patients in the ITT population obtained SD (10%), and 19 patients (47.5%) developed PD. Thirteen patients (32.5%) were nonevaluable since they did not have follow-up evaluation or did not complete twelve weeks of therapy without progression. Clinical benefit (CR + PR + SD) was seen in 8 patients (20%). One CR patient received treatment with ANP after he relapsed after surgical resection, RT and chemotherapy with Gliadel® wafers. This patient developed recurrence after 2.5 years of CR after ANP treatment and underwent surgical resection. His tumor transformed to primitive neuroectodermal tumor (PNET) for which he received further treatment (Figure 1). He is currently well and alive over 12 years after treatment start. Another CR case came for treatment with ANP after his tumor recurred after two different types of RT and TMZ. Among two PR cases, one developed tumor recurrence after surgical resection and two different types of RT, and another patient was treated with ANP after surgical resection, RT and chemotherapy. In RGBM group, SD was 3.3%, PD was 50%, and NE was 33.3%. In ERGBM group, SD was 4.2%, PD was 58.3%, and NE was 20.8%.
In the ITT group, OS was 48.9% at 6 months, 28.3% at 1 year and 2.6% at two, five and ten years. The PFS at six months was 17.5%. In the RGBM group, OS was 55.5% at 6 months, 34.7% at 1 year and 3.5% at two, five and ten years. The PFS at six months was 16.7%. In the ERGBM group, OS was 65.5% at 6 months, 39.3% at 1 year and 4.4% at two, five and ten years. The PFS at six months was 20.8% (Figure 2 and Figure 3).
Table 3 compares the results of the current study to other select studies.
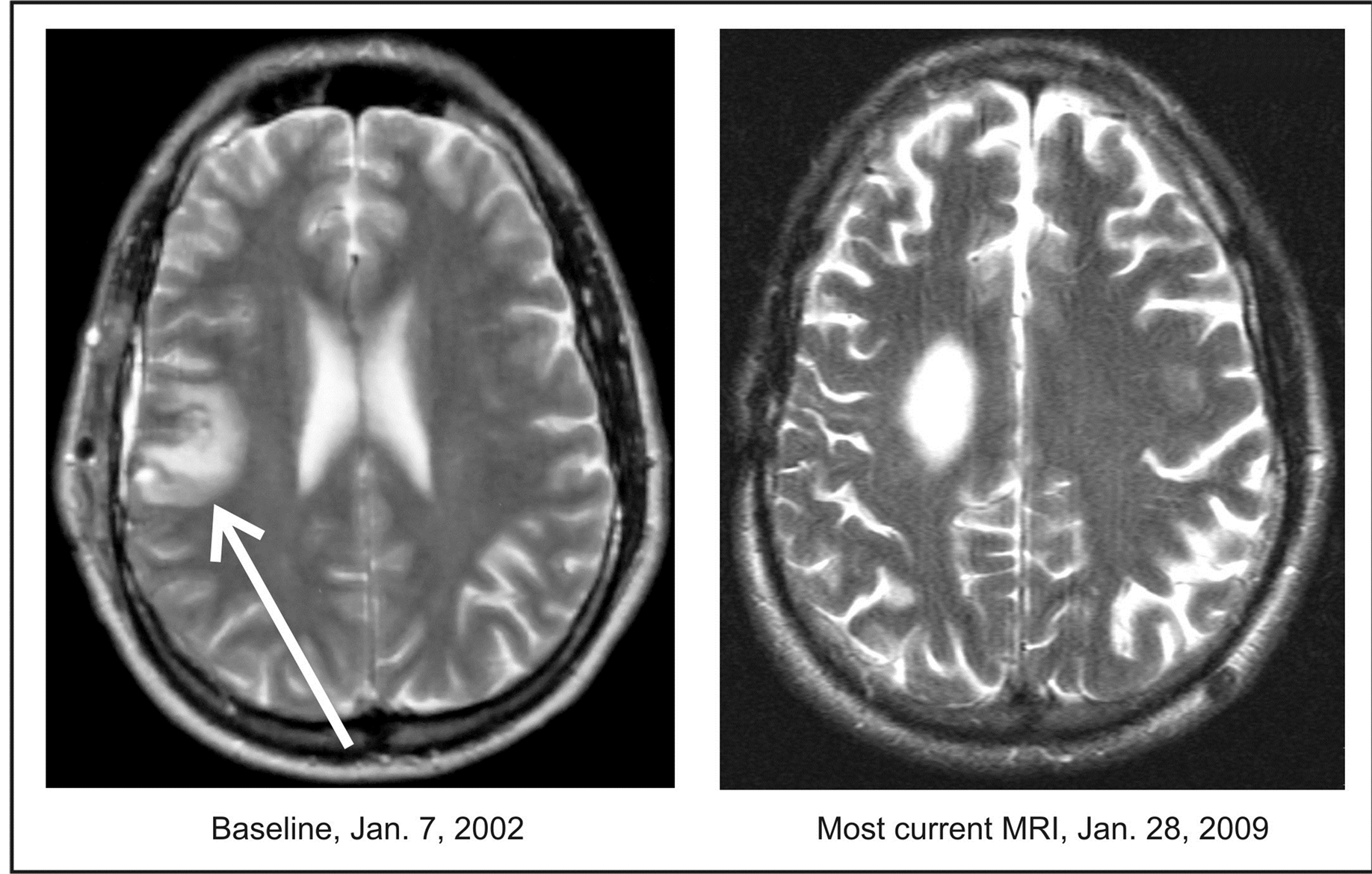
Figure 1. Patient 28. A 42-year-old male diagnosed with a GBM developed recurrence after gross total resection, RT and chemotherapy with Gliadel® wafers. The patient was admitted to the Phase II study on January 7, 2002 and discontinued treatment on March 1, 2003. The CR was documented on February 13, 2002. OS is over 12 years.

Figure 2. The Kaplan-Meier survival curve. Overall survival from the treatment start.
8. Safety and Adverse Events
Toxicity
The following serious adverse drug events (ADEs) were observed during the course of treatment in the ITT group: hypernatremia 12.5%, hypokalemia 7.5%, fatigue 5%, and somnolence 2.5%. All serious ADEs were observed in a group of seven patients. Generally, the treatment was well-tolerated with reversible toxicities. There were no chronic toxicities. All adverse events (AEs) have been coded according to Common Terminology Criteria for Adverse Events v3 (CTCAEv3). Table 4 compares incidence of ADEs in this study to other studies of chemotherapy for RGBM. It is planned to decrease the maximum dosage of ANP A10 in future studies to 12 g/kg/d to reduce the incidence of the most important ADEs: hypernatremia, hypokalemia, fatigue and somnolence.
9. Discussion
Clinical trials in RGBM have been extensively reviewed [19] . Unfortunately, at recurrence only a small number of patients were eligible for second surgery or RT. In patients who received prior TMZ, a PFS at 6 months is between 20% - 30% after retreatment with TMZ in different dosing regimens, nitrosoureas, or bevacizumab. Regimens with newer targeted therapy did not produce evidence of greater activity, but were usually associated with more toxicity. The treatment with ANP for RGBM and ERGBM was compared with the results of four other Phase II studies in which GBM recurred after RT and chemotherapy with TMZ (in one study TMZ and bevacizumab) (Table 3).
Vredenburgh et al. conducted Phase II of bevacizumab and irinotecan in recurrent malignant glioma including 23 patients with GBM. He reported 4% of CR, 56% PR and 38% SD [25] . Despite remarkable objective responses, 6-month PFS was 30%, median PFS 4.6 months, and median OS 9.3 months. The toxicity was substantial and 9% of patients died as the result of ADE (Table 4).
Reardon et al. reported the results of Phase II study of carboplatin, irinotecan and bevacizumab for recurrent GBM after progression on TMZ and bevacizumab [26] . Nearly all patients were enrolled while progressing on bevacizumab therapy. There was no CR or PR, but 80% of patients had SD. PFS at 6 months was 16%, median PFS 2.3 months, and median OS 5.8 months. A substantial percent of patients experienced thrombocytopenia and neutropenia. Desjardins et al. conducted treatment with daily TMZ for recurrent GBM in 32 patients [27] . The authors reported 28% PR and 30% SD; however, PFS at 6 months was 18.8%, median PFS was 3.6 months, and median OS only 8.6 months. One-year survival was 31% and two-year survival 8%. The toxicity was tolerable, but there was one treatment-related death. Omura et al. reported the results of Phase II study of continuous low-dose TMZ in 28 patients with RGBM who relapsed during prior treatment [20] . There was no CR, but 11%

Table 3 . Selected Phase II clinical studies in recurrent glioblastoma multiforme in comparison to results of current study.
CR—complete response; ERGBM—eligible recurrent glioblastoma multiforme; OS—overall survival; PR—partial response; PFS—progression-free survival; RGBM—recurrent glioblastoma multiforme; SD—stable disease; TMZ—temozolomide. *28 evaluable patients, 37 included in survival analysis; **The additional 30% of patients were treated with RT only and 3% of patients underwent surgery only; ***The additional 12.5% of patients were treated with RT only.

Table 4. Incidence of adverse drug experiences (ADE), grades 3 and 4 and treatment related deaths reported in antineoplastons study (ITT) compared to the studies of chemotherapy for recurrent glioblastoma multiforme.
PR and 25% SD. The survival data indicated PFS at 6 months 19%, median PFS of 2 months and median OS of 7 months. One-year OS was 35%. The treatment was associated with tolerable toxicity, but 30% of patients developed lymphopenia.
10. Conclusion
The study reached the goal with 4 cases of CR and PR in the ITT population. The data acquired in patients with RGBM present improved survival outcomes due to ANP therapy, and tolerable toxicity. The future studies will contain control groups.
Acknowledgements
The authors express their appreciation to the additional physicians involved in the care of the patients: Drs. Robert A. Weaver, Robert I. Lewy, Eva Kubove, and Barbara Szymkowski. Preparation of the manuscript was provided by Carolyn Powers, Adam Golunski, and Jennifer Pineda.
NOTES
*Corresponding author.