Blueberry: Functional Traits and Obtention of Bioactive Compounds ()
1. Introduction
Color is associated to many aspects of our lives, shifting consciously or not the way we feel and decide about everyday situations, including those involving the preference for a specific food. The appearance, safety, acceptability and sensorial features of foods are all affected by color. Thus, the industry uses colors to help consumers to select foods and beverages, and this is also the way consumers notice and judge food quality [1] . Natural dyes are usually less stable and more expensive than synthetic ones, but their study and use are increasingly attracting the attention of both industry and research centers worldwide. Currently there is demand from consumers for a more natural food composition. In addition to this trend for exchanging synthetic for natural dyes, due to their association with food quality and health, there is an increasing limitation of synthetic dyes for use in industry [2] due mostly to its association with adverse reactions in some consumers, and toxicological, mutagenic or carcinogenic effects [3] .
Fruit quality depends greatly on the technology used both in harvesting and post-harvest managements. Methods employed in these two stages do not improve fruit quality, but help reducing processes of senescence and decomposition, ensuring better conservation and thus providing a longer shelf life [4] .
Dehydration, both by atomization and liofilization, result in foods with higher nutritive value, stable and versatile, that can be used as flavorings, colorings, sweeteners, vitamins, minerals, acidulants, spices and drugs [5] .
Blueberry is a fruit plant native from temperate regions, included in the family Ericaceae and genus Vaccinium. In average, fruits are 1 cm in diameter weighting 1.5 g and can be both processed or consumed in natura [6] . Its fruits are deep blue in color with sweet acid taste, presenting a high number of small sized seeds.
Blueberry is economically important especially in the North America and Europe, centers of origin of the species in this genus. The interest in this crop in other regions has been increasing lately [7] . Among the studied small fruits, blueberry is one of the richest in antioxidants, showing particularly high amounts of polyphenols both in the pulp and peel, whose function in the fruit is to protect cell walls [8] . Anthocyanins are found in higher amounts in the peel, and lots with smaller fruit size present a higher total surface area, thus resulting in higher antioxidant content by weight, when compared to the extraction from larger fruits [9] .
Anthocyanin content in blueberry is affected more by genotype than by climatic or environmental factors. Cultivars differ in anthocyanin content, pH, acidity, solids and moisture content, fruit taste, fruit size, number and size of seeds, among others [10] . Rocha [11] points out that the variety grown differs in distinct regions of Brazil, mainly as a function of better adaptation to local environmental conditions.
Anthocyanins, like other natural dyes, present stability problems when exposed to light, pH and oxygen. Degradation occurs since extraction and purification to processing and storage, and the main factors affecting stability are the chemical structure of the pigment, pH, temperature and type of solvent used for extraction [12] .
In this context, due to the importance of blueberry as a source of natural pigments, as well as its high added value due to the association with functional food, it is important to develop and enhance methods of extraction and stabilization of anthocyanins from blueberry, which would result in higher post-extraction stability of the pigment.
This review aimed to expose the potential of blueberry (Vaccinium myrtillus) as a source of natural dye, associated with its properties of functional food.
2. Market of Fruits and Pulps
Brazilian agribusiness has an efficient, modern and competitive fruit production chain. Brazil is the world’s third pole of the fruit industry, after China and India, with annual production of about 38 million tons. In 2006, exports of fresh fruits (except oranges) reached United States Dollar (USD) 471.8 millions, an increase of 95% compared to USD 241 millions in 2002. From January to April 2007 there was an increase of around 50% in the value of exports, compared with the same period of the previous year, from USD 102.3 to USD 150.1 millions [13] .
Blueberry, despite being a species of recent introduction in Brazil, is widely grown in the Northern Hemisphere, especially Europe and the United States. In these regions, the species has great commercial importance and its benefits as functional food are largely explored.
The United States holds 50% of world production of blueberry, being the state of Maine the largest producer, with 25% of US production. Canada (provinces of Quebec and Nova Scotia) produces 33% and 16% is produced in the European continent, leaving the rest of the world with a share of only 1% of the amount produced in 2002 [14] .
It is also in the United States where the highest rates of consumption are observed. The US imports about 82% of the production from the rest of the world. The high demand of these markets has driven cultivation in non-traditional regions such as South America, especially in countries like Chile, with 2500 ha, Argentina, with 1500 ha, and Uruguay with 200 ha, aiming to an out-of-season supply of those great markets [15] .
The first export from Argentina, whose destination was the United Kingdom (UK), occurred in 1994, but only in 1997 this country began to export to the US market. Now producing about 380 tons per year, 74% of this production is aimed to supply the US market between the months of October and December [14] .
The area planted with blueberry in Brazil is more than 150 hectares, nearly all aimed for export. Rio Grande do Sul is the state that stands out in the production of blueberry. In 2008, 45 farmers cultivated an area of 65 ha producing 150 tons [15] . The largest consumer market in Brazil is the state of São Paulo, and the place with the largest number of wholesalers of this fruit is the Sao Paulo Warehouse Terminal (ETSP), owned by the Company of General Warehouses of São Paulo (CEAGESP). Virtually all production is sold in natura, and a small part industry is intended for juice, ice cream and sweets [15] . As a noble and high valued fruit, the commercial use of blueberry in industrialized products tend to increase the variety of uses and availability of this fruit while supplying consumers equal benefits they would have consuming in natura fruits [11] .
3. Fruit Traits and Production
Blueberry is a shrub fruit tree from Temperate Climates, with erect or prostrate growth habit, from the family Ericaceae genus Vaccinium [10] , producing a dark blue flattened berry fruit, crowned by persistent calyx lobes, with many seeds surrounded by a whitish pulp of sweet-acid taste [6] . The plant is hexaploid, with deciduous leaves in winter, 2 - 4 m high, which requires a variable number of chilling hours per year with temperatures below 7.2˚C, depending on the genotype. Shows cartaceous leaves, glabrous, 4 - 8 cm in length. Flowers are formed during the spring when plants are still with no leaves [16] . In general, fruits are about 1 cm in diameter weighting 1.5g and may be intended both for fresh consumption and for processing in the form of puree, fruit juice, jam, cakes, muffins, snacks and cereals [6] [10] .
The main commercially important species can be classified into three groups according to genotype, growth habit, fruit type and other traits, “highbush” has the best ranking in size and taste, “rabbiteye” produces fruits with smaller size and lower quality, but has a higher yield per plant and better post-harvest conservability and “lowbush” that produces fruits of small size, generally intended for industrial processing [10] [17] . The richness in anthocyanin pigments, substances of high antioxidant power and preventative of degenerative diseases, the unique flavor and the color are distinctive factors in blueberry which attract consumers [18] .
In Brazil, the main planted cultivars of blueberry belong to the group “rabbiteye”, being characterized by high vigor, long lived plants with high yield, tolerance to heat and drought, low demand of cold for dormancy break, early flowering, long period between flowering and maturity and firm fruits with long shelf-life, since it is preserved properly. Another feature is the lower requirement of chilling hours (below 7.2˚C) than varieties of the group “highbush”. They are able to grow and flourish with only 360 hours of chilling, while those in the group “highbush” need between 650 and 800 hours of chilling [10] . Silveira et al. [19] found that even the source of pollen is important for the quality of fruits of the variety “rabbiteye” produced in the Southern region of Brazil. In addition, Rocha [11] remarks that the variety Bluegem, from the group “rabbiteye”, is one of the most widely grown in the Southeastern region of Brazil due to its wide adaptation and lower cold requirement.
Among the limitations of cultivars from the group “rabbiteye”, it can be highlighted the full color of the fruit before the ideal time for harvest—where fruit would present the best quality and taste; a tendency to crack the fruit skin under wet weather conditions and the long period to reach maximum yield [20] .
Brazil is a relatively new producer of blueberry. The first experiments started in 1983 under the responsibility of Embrapa Temperate Agriculture, at the city of Pelotas, state of Rio Grande do Sul, with the introduction of the collection of cultivars with low chilling requirement of the group “rabbiteye”, coming from the University of Florida, United States. In Rio Grande do Sul state, the region of the city of Vacaria pioneered the commercial production of blueberry being currently a reference in terms of production. Nowadays, cultivation is expanding in the country, especially in the temperate region, where there is great demand for cultivars adapted to regional climate conditions [21] .
4. Phenolic Compounds
Phenolic compounds are defined as substances having an aromatic ring with one or more hydroxyl substituents [22] . The beneficial properties of these compounds can be attributed to its ability to capture free radicals due to oxidation-reduction properties, playing an important role in the elimination and deactivation of these radicals [22] [23] .
Many phenolic compounds of foods are soluble in water and other organic solvents. Phenolic compounds found in foods generally belong to the class of phenolic acids, flavonoids, lignins, stilbenes, coumarins and tannins [24] . For quantification of total polyphenols, the Folin-Denis method is the most suitable for plant materials and beverages, because there is no influence of interferents such as proteins, sugars and other reducing substances other than polyphenols, and therefore results are more accurate. This method is based on reducing the phosphomolybdic-phosphotungstic acid (Folin-Denis reagent) to a blue colored complex in alkaline solution, by the phenolic compounds. It is also a reference method used for determination of total phenolic compounds by the Association of Official Agriculture Chemists [24] [25] .
The interest in phenolic compounds in foods reached new heights in recent years. Subtracting the usual academic interest in biology and chemistry discovery and identification of phenolic compounds in nature, it is being noticed that science, along with commercial interests, is looking into enhancing studies and working on these compounds in order to add greater value to foods, as well as to provide beneficial effects to health [26] . Blueberry (Vaccinium sp.) presents in its composition high concentrations of phenolic compounds [27] , being higher than in most other fruits [28] .
Sellappan et al. [29] found phenolic acids gallic, p-hydroxybenzoic, p-coumaric, ferulic and caffeic in blueberries produced in the state of Georgia (US). Ellagic acid was found in some of the evaluated cultivars. From the group of non-anthocyanic flavonoids, the flavanols catechin and epicatechin were found, and as representatives of flavonols, quercetin, myricetin and kaempferol were found.
Taruscio et al. [30] evaluated the phenolic composition (phenolic acids and non-anthocyanic flavonoids) of blueberry cultivars belonging to nine species of the genus Vaccinium. The (-)-epicatechin was the predominant flavanol, followed by catechin. However, in some species catechin was not detected (<5 μg∙g−1). From the group of flavonols were identified and quantified quercetin and myricetin, but the presence of kaempferol was not detected. In that study, chlorogenic acid was found as a major phenolic component in all cultivars.
Zadernowski et al. [31] identified 17 phenolic acids present in some varieties of blueberry by gas chromategraphy (GC) coupled to mass spectrometry (MS). The benzoic acids found were: gentisic, gallic, o-pyrocatechuic, protocatechuic, salicylic, syringic, vanillic and veratric. As representatives of cinnamic acids, caffeic, mcoumaric, o-coumaric, p-coumaric, 3,4-dimethoxycinamic, ferulic, sinapic and hidroxycaffeic acids were found.
Zheng and Wang [32] identified a high number of phenolic compounds present in blueberry. The differential of this study was the determination of these compounds in glycosylated form, i.e., without applying the hydrolysis step. Thus, it were found the flavonols myricetin-3-arabinoside, quercetin-3-galactoside, quercetin-3-glucoside, quercetin-3-arabinoside, quercetin-3-rhamnoside and kaempferol derivates. The anthocyanins found were delphinidin-3-galactoside, delphinidin-3-glucoside, delphinidin-3-arabinoside, cyanidin-3-arabinoside, cyanidin- 3-galactoside, cyanidin-3-glucoside, cyanidin-3-xyloside, petunidin-3-galactoside, petunidin-3-glucoside, petunidin-3-arabinoside, malvidin-3-galactoside, malvidin-3-glucoside, malvidin-3-arabinoside, peonidin-3-galactoside, peonidin-3-glycoside and peonidin-3-arabinoside.
4.1. Anthocyanins
Anthocyanins (from the Greek “anthos”—flower, and “kyanos”—blue) [33] are a group of water-soluble plant pigments [23] and chemically they are flavonoid phenolic compounds, widely distributed in nature, responsible for the color of fruits, flowers and vegetables [34] .
The use of anthocyanins as colorants occur since the Roman Empire, when Romans used highly colored fruits to enhance the color of wine [35] . Anthocyanins are known as alternative sources to synthetic dyes red in color. Besides color attributes, interest in these compounds has been intensified due to their beneficial effects to health [34] [36] . Its spectrum comprises several colors ranging from red to blue, resulting in shades of purple. Many fruits, vegetables, leaves and flowers owe their attractive color to these pigments, which are dispersed in cell vacuoles [23] .
The structure of anthocyanins is based on a C15 skeleton consisting of an aromatic ring linked to a second ring in the position C2. This structure is supplemented by one or more linked sugar molecules connected to distinct hydroxylated positions in the basic structure [36] . When sugar molecules are absent, they are called anthocyanidins (aglycons) (Figure 1). There are six anthocyanidins most commonly found in natural pigments, being most of them substituted in hydroxyl positions 3 and 5 [34] .
Anthocyanins are methoxyl/polyhydroxy glycosylated salts derived from 2-phenyl-1-benzopyrylium (flavylium cation) [34] . Anthocyanins differ among them by the number of hydroxyl groups, number and type of sugars attached to the molecule, position of the sugar and the number and nature of the aliphatic or aromatic acids attached to the sugars of the molecule [38] . Figure 2 illustrates the structure of an anthocyanin.
The most frequently found anthocyanin is cyanidin, which confers the red color [40] . The vast repertoire of colors displayed in the range between red and blue, is the result of the complex formed between these polyphenols, pectins and metallic ions [41] . The main types of anthocyanins present in nature are shown in Table1
The solubility of these compounds in water allows its incorporation in several aqueous food systems, which makes anthocyanins attractive natural colorants [42] . However, the use of anthocyanins, particularly as natural colorants, is hampered by poor light stability at the ranges of pH commonly found in foods (weakly acid pH) [43] [44] .
Another problem with the color change is the flavylium cation, highly reactive due to the deficiency of electrons, being susceptible to attacks by nucleophilic reagents such as water, peroxide and sulfur dioxide [45] . These reactions, in general, result in discoloration of the pigment and are often undesirable when processing fruits and vegetables. Bleaching is one of the major contributors to the loss of anthocyanins in foods, especially if accompanied by the addition of sulfites or sulfur dioxide. The addition of such substances results in rapid discoloration of anthocyanins which become yellowish [23] . According to Dyrby et al. [46] , a rise in temperature causes logarithmic increase in the degradation of anthocyanins, with the appearance of the molecular form chalcone. In Figure 3 some changes that occur in anthocyanins are presented.
Although temperature is a critical factor for stability of anthocyanins, novel heating systems of blueberry pulp allowed lower losses of this pigment than conventional heating systems. Sarkis [47] created a prototype system, which comprises passing electrical current through the pulp of blueberry instead of the classical fire heating. The prototype system was capable to efficiently heat blueberry pulp with anthocyanin losses between 5.71% and
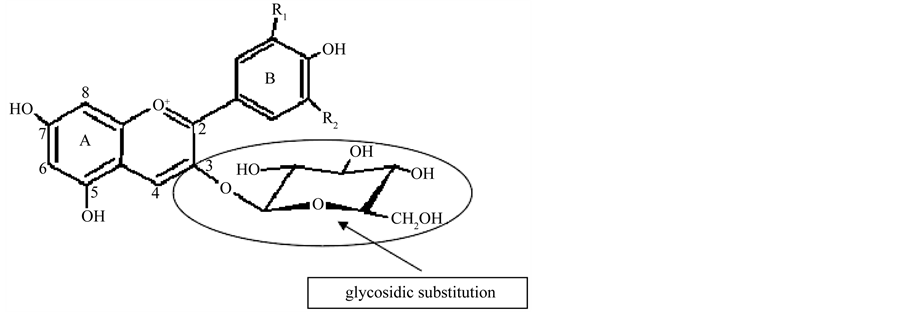
Figure 2. Structure of an anthocyanin. Source: adapted from [39] .

Table 1. Anthocyanins most commonly found in nature and their respective color.
Source: adapted from [38] .
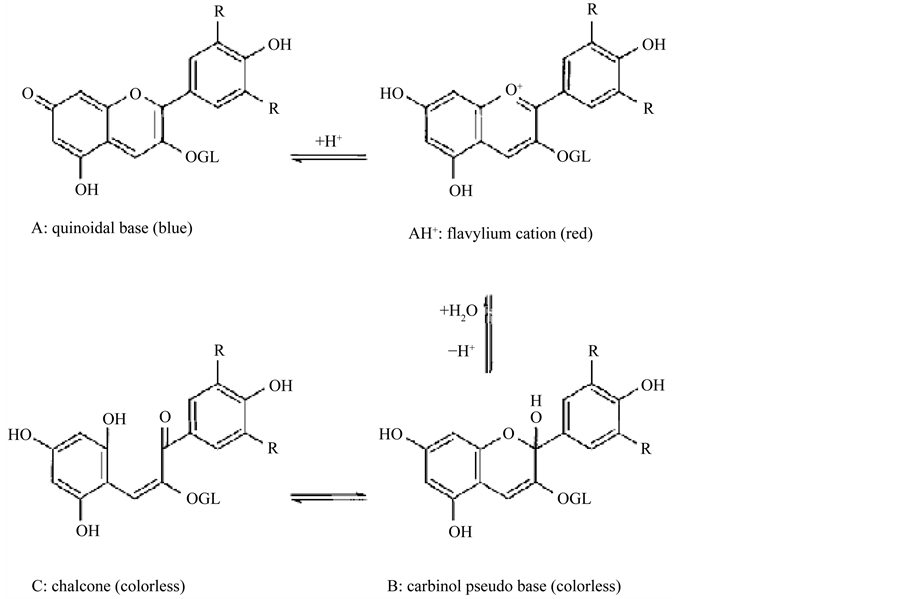
Figure 3. Structural changes in anthocyanins. Malvidin-3-glucoside at 25˚C. Source: [35] .
14.67%, depending mainly on the amount of electrical tension to which the food was submitted. The author reports that higher tensions resulted in higher losses than usual heating systems, but lower tensions allowed adequate heating with lower anthocyanin losses compared to usual heating systems.
Due to the wide distribution of anthocyanins in the plant kingdom and its expressive color, many plants have been studied as potential sources. However, only two, grapes (Vitis vinifera) and red cabbage (Brassica oleracea), have reached commercial success so far. Francis [48] also cites Sambucus nigra, Aronia arbutifolia and Daucus carota L. as short-term potential sources. In this sense, it is essential to identify new sources that are economically viable.
In fruits, anthocyanins are located mainly in the peel and occasionally in the pulp, and in some cases, contain only one type of pigment, as observed in apple (Pyrus mallus) and purple gooseberry (Ribes rubrum), which contain only cyanidin. Furthermore, fruits such as grape and blueberry have a combination of five to six anthocyanins [49] .
Pertuzatti et al. [50] evaluated the anthocyanin content in whole fruits, peel and pulp of the varieties of Blueberry “Powder Blue” and “Delite”. Powder Blue showed the highest levels of anthocyanins in the fruit and peel, with average values of 256 and 716 mg of cyanidin-3-glucoside/100g of fresh fruit, respectively. The variety Delite showed lower average values, between 144 and 382.3 mg/100g for fruit and skin, respectively. In the pulp, the variety Delite showed the highest levels of anthocyanins, about 80.9 mg of cyanidin-3-glucoside/100g, while the levels were 2.7 mg/100g of pulp of the variety Powder Blue. This is due to the fact that the pulp of the variety Delite has reddish color while Powder Blue has yellowish fruits. Rocha [11] found 58.9 and 1182.0 mg of total anthocyanins, respectively in the pulp and extract of blueberry from the variety Bluegem, characterized for its reddish color.
In a notable work [51] , anthocyanins were identified and quantified in several cultivars of blueberry produced in Australia. All cultivars presented a similar qualitative profile for anthocyanins, but the proportion of each anthocyanin varied depending on cultivar. Cultivars from the species Vaccinium ashei presented total anthocyanin contents significantly higher than that of the species Vaccinium corymbosum. The evaluated cultivars were Crunchie, Star and Sharpe (“highbush” group, Vaccinium corymbosum), Climax, Brightwell and Powder Blue (“rabbiteye” group, Vaccinium ashei). Fifteen anthocyanins were identified. The major anthocyanidins were delphinidin, petunidin and malvidin.
Vendramini and Trugo [52] identified aglycones of anthocyanins in acerola (Malpighia punicifolia L.). Anthocyanins content in the skin of mature acerola was 37.5 mg∙100g−1. Lima et al. [53] determined the total anthocyanins in acerola from the varieties Barbados, Coopama, White Flower, Inada, Miró and Okinawa. The selections Inada and Barbados had the highest levels of these pigments, compared to the other selections, making them agriculturally interesting as sources of anthocyanins.
Mota [54] evaluated the composition of anthocyanins in six cultivars of black berry (Rubus brasiliensis), finding a predominance of cyanidin. During the preparation of juice with these fruits, there was a decrease of 42% in the anthocyanins content. The storage time also contributed to this decrease, which was lower in samples kept under refrigeration, compared to the fresh juice.
4.2. Antioxidant Activity
Antioxidant activity can be defined as the ability of a compound to inhibit oxidative degradation. It can be assessed by the antioxidant potential, which is determined by the composition and properties of the constituents and, moreover, by the biological activity, which depends on the bioavailability of the antioxidant [55] .
There are several definitions for “antioxidants”. The broader definition is that they are “substances present in low concentrations, compared to the oxidizable substrate, which significantly delay or inhibit oxidation of the substrate” [56] . There is also the definition of “food antioxidant”, which according to [56] “is any substance in the diet capable of significantly reducing the adverse effects produced by reactive species, such as those of oxygen and nitrogen, and have normal function in the body”.
The term “free radical” is often used to designate any atom or molecule having one or more unpaired electrons in the outermost orbitals, which makes these highly reactive molecules able to react with any nearby compound, presenting an oxidizing role in the reaction [57] . Thus, the formation of reactive oxygen species (ROS) and free radicals occurs in the normal metabolism, which raises the need for inactivation of these highly reactive molecules. The imbalance between oxidant and antioxidant molecules results in the so-called oxidative stress [58] , which affects many biological molecules including lipids, proteins, carbohydrates and deoxyribonucleic acid (DNA). Consequently, reactive oxygen species (ROS) are implicated in several human degenerative diseases such as cardiovascular disease, cancer and cognitive dysfunctions [59] .
To combat the so-called free radicals or reactive oxygen (ROS) and nitrogen (RNS) species, the body possesses an effective defense system (endogenous antioxidants), which includes several enzymes and antioxidant molecules of high and low molecular weight [8] . This protection can be based on multiple mechanisms of action, mainly the inhibition of the generation and the ability to neutralize ROS/RNS, reductive capacity, ability to chelate metals, antioxidant enzyme activity and inhibition of oxidative enzymes [60] . In addition to these endogenous antioxidants, there are those consumed in the diet (exogenous antioxidants), which include ascorbic acid (vitamin C), vitamin E, vitamin A, carotenoids and phenolic compounds.
Among the antioxidants present in fruits and vegetables, the most active and frequently found are phenolic compounds such as phenolic acids and flavonoids [61] . Several methods are used to determine the antioxidant activity of extracts and isolated compounds, being one of the most commonly used the evaluation of the capturing activity of the free radical 2,2-diphenyl-1-picryl-hydrazyl-DPPH•, purple in color, with absorption band around 515 nm. By the action of an antioxidant (AH) or a radical species (R•), the DPPH• is reduced changing to yellow color, with consequent disappearance of the absorption; so, this process is possible to be monitored as a function of absorbance levels. From the results, it is possible to determine the percentage of antioxidant activity or free radical capturing, and/or the percentage of DPPH• remaining in the reaction medium at a given time [62] [63] .
The antioxidant activity also varies as the fruit is processed. Rocha [11] showed that blueberry from the variety Bluegem presented 32.5% of its antioxidant activity in the fresh pulp, while its activity increased to 42.88% in the extract obtained by the previously described DPPH method. The same author studied methods to transform both pulp and extracts to powder forms, increasing its viability to be used as natural colorants in industrialized foods while keeping its antioxidant activity.
4.3. Factors Which Affect Phenolic Compounds and Antioxidant Activity
The phenolic content of blueberry (Vaccinium sp.) presents great qualitative and quantitative variation. Several studies have shown that this variation is dependent on intrinsic factors—genus, species and cultivar—and extrinsic—environmental, cultivation, handling and storage conditions [64] [65] . In addition, factors such as the complexity of phenolic compounds, methods of extraction and quantification, can also affect the composition of this group of compounds [11] .
Prior et al. [66] studied four species of blueberry (Vaccinium corymbosum, Vaccinium ashei Reade, Vaccinium angustifolium and Vaccinium myrtillus), reporting an increase in the concentration of phenolic compounds and antioxidant activity during maturation for varieties Brightwell and Tifblue. In contrast, the region of cultivation (Oregon, Michigan and New Jersey (US)) had no influence on the phenolic content and antioxidant activity of the cultivar Jersey. Connor et al. [67] evaluated the influence of cultivar, location and growing season on the phenolic content and antioxidant activity of blueberry, and concluded that, contrary to the findings of [66] , there was interaction between genotype and environment in determining the antioxidant activity of blueberry. Rodrigues et al. [68] also found that the concentration of phenolic compounds and its antioxidant activity was dependent on both variety and production location, for the varieties Bluecrop and Tifblue.
Castrejón et al. [69] observed a decrease in phenolic compounds (hidroxicinamic acids and flavonols) and antioxidant activity of blueberry cultivars from the species V. corymbosum during ripening. The values for total phenolic content ranged from 60.76 to 33.00 mg∙100g−1 (expressed in gallic acid—EGA) of dry weight, from the initial stage to the end of ripening, respectively.
The influence of planting system (organic or conventional) on the phenolic composition and antioxidant activity of blueberry was analyzed by [64] . Results from this research showed that blueberry (Vaccinium corymbosum cv. Bluecrop) produced in the organic system had higher concentration of phenolic compounds, anthocyanins and antioxidant activity than those produced in the conventional chemical-based system.
Kechinski [70] studied the inactivation of two of the main enzymes responsible for degrading anthocianins from extracts of blueberry—polyphenol oxidase and peroxydase, and concluded that the activity of the former was high at 40˚C and almost null at 80˚C, which led the author to establish a thermal treatment at 80˚C for 219 seconds to reduce as much as possible the activity of the enzyme polyphenol oxidase. For the latter, a combination of strainer and thermal treatment of the extract resulted in significant reductions in the activity of the enzyme.
The conditions and time of storage of the extracts are also important factors for the total phenolic content and antioxidant activity measured. Srivastava et al. [71] evaluated the effect of temperature (−20˚C ± 1˚C, 5˚C ± 1˚C, 23˚C ± 1˚C and 35˚C ± 1˚C) and storage time (15, 30, 45 and 60 days) on total phenols and antioxidant activity of blueberry extracts, and found that there was no significant loss (p < 0.05) during 30 days of storage for phenolic compounds, anthocyanins and antioxidant activity at −20˚C. However, losses were observed when storage occurred in temperatures between 5˚C and 35˚C. Besides storage temperature, Rocha [11] reports that the water activity in the powdered extracts also caused changes in the maintenance of the antioxidant activity of blueberry variety Bluegem.
4.4. Functional Properties
Epidemiological and in vitro studies suggest that blueberry (Vaccinium sp.) helps maintaining the health and acts as a barrier to the effects of aging, particularly with respect to neurodegeneration and cognitive defects. There is also evidence of its action in the prevention of cardiovascular diseases and some types of cancer [72] . Many beneficial effects seem to be related to the antioxidant properties of phenols present in the fruit [73] [74] .
Duffy et al. [72] conducted a study with rats, verifying that supplementing the diet with 2% of blueberry extract for 8 weeks protected them against neurodegeneration and cognitive defects, mediated by excitotoxicity and oxidative stress. This research supplied evidence that supplementation with blueberry extract can be used for preventing or treating Alzheimer’s disease, and possibly other neurodegenerative disorders, being also suggested that the extract attenuates degenerative processes caused by oxidative or inflamatory processes.
Wolfe et al. [28] evaluated the antioxidant activity of 25 types of fruits commonly consumed in the US, and found blueberry as one of the fruits with higher antioxidant activity in cell culture system. It was also observed a high correlation between total phenolic content and the antioxidant activity in cells, showing that the former can be used as an indicator of antioxidant activity. These results indicate the possible effects of blueberry extracts on attenuation of oxidative events in cells, thus decreasing the risk of cancer.
Seeram et al. [75] investigated the property of blueberry extracts in inhibiting the proliferation of tumor cells in the oral cavity, breast, colon and prostate, concluding that the action was dose-dependent, and distinct among cell types. Furthermore, the extracts stimulated apoptosis in cultures of colon cancer cells.
Boivin et al. [76] evaluated the effect of blueberry juice on cell death and cell cycle interruption in human cancer cells of the stomach, breast, prostate and intestine. These authors found a high capacity of the juice in inhibiting cell growth, especially for the juice of fruits from cultivars in the group “lowbush”. The mechanism of action appears to be related to cell cycle interruption, more than caspase-dependent apoptosis.
The action of phenolic compounds of blueberry in the reduction of one of the risk factors of cardiovascular disease was demonstrated by [73] . An experiment with swines showed that supplementation with blueberry extract (Vaccinium corymbosum cv. Jersey) reduced the levels of total cholesterol, LDL and HDL. The greatest decrease was observed with blueberry at concentration of 2%, and total cholesterol, LDL and HDL were reduced, respectively, in 11.7%, 15.1% and 8.3%.
5. Conclusion
Due to the various beneficial effects in the body related to the continued consumption of blueberry, both in natura and as byproducts such as natural anthocyanic pigments, researches should be directed to the technical feasibility of obtaining these pigments from blueberry, as well as to reduce costs with extraction of this pigment. Brazil has the potential to have a significant share in exports of this fruit, whose main targets are parts of the world whose population has higher income and education level, as the North American and European countries. Some pioneering studies with blueberry have been conducted in Brazil, but the intensification of researches will supply the missing pieces to maximize productivity, increase levels of anthocyanic pigments in fruits produced, as well as the stability of these compounds after extraction. Another focus of research should be the use of natural pigments in processed products aiming to replace those currently used.
NOTES
*Corresponding author.