Cations and Anions in Sewage Sludge from Gaza Waste Water Treatment Plant ()
acid method (orthophosphate), titrimetric method (Cl−), inductive coupled plasma analyzer (ICP, heavy metals). All the processes of experiments and analyses were described clearly for reference. Results showed that concentrations of Na+, K+, Ca2+ and Mg2+ were 28.93, 2.53, 271 and 177 mg/kg
respectively whereas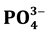 ,
, 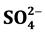 ,
, 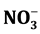 , and Cl− were 0.434, 18.59, 0.87 and 0.026 g/kg respec-
, and Cl− were 0.434, 18.59, 0.87 and 0.026 g/kg respec-
tively. The concentrations of Fe, Cu, Pb, Zn and Mn were 125.12, 172.56, 76.88, 218.73 and 157.56 mg/kg respectively. These results indicate that sewage sludge from Gaza contained high fractions of most plant nutrients accordingly, and it may be advantageous to use the sludge as a natural source of plant fertilizers.
Keywords:
1. Introduction
Gaza Strip (GS) has a coastline of 40 km at the eastern extreme of the Mediterranean and on the edge of the Sinai Desert. GS has a total area of 365 square kilometers and the population is estimated to be around 1,701,437 people [1] .
Sludge (biosolids) may be defined either the matter which refers to the residual, semi-solid material left from industrial wastewater, sewage treatment plants or settled suspension obtained from conventional drinking water treatment and numerous other industrial processes [2] . Sludge may be dried and incorporated with some carbonaceous materials to produce a suitable composted material for the use in the agriculture to increase the production and improve the soil properties. Chemical and biological compositions of sewage sludge depend on the wastewater composition [3] . Usually, it is rich in Organic Mater (OM) and plant nutrients such as Nitrogen (N), Phosphorus (P) and Calcium (Ca) [4] and can improve soil physical, chemical and biological properties, such as porosity, aggregate stability, bulk density, soil fertility, water movement and retention [5] . There are four wastewater disposal and treatment facilities in Gaza Strip, Beit Lahia, Gaza City, Khan Younis, and Rafah waste-wa- ter treatment plants. These stations are not sophisticated treatment technology; they consist of anaerobic lagoons, aerated lagoons and maturation ponds. It is expected that large amounts of sludge are being produced from Gaza Strip wastewater treatment plants.
Furthermore, Casado-Vela et al. [6] monitored the effect of the application of three increasing amounts of composted sewage sludge (3, 6 and 9 kg・compost・m−2) on the physicochemical properties of a horticultural calcareous soil where two types of plants were grown under two exploitation regimes (one in a greenhouse and the other in open-air). They found out that the 9 kg・compost・m−2 application promoted the appearance of deleterious effects on the properties of soil, such as salt accumulation, a significant increase in the electrical conductivity and an input of heavy metals (Pb > Cr > Cd). The 6 kg・compost・m−2 application provided a supply of nutrients necessary to grow peppers plants under both exploitation regimes.
In a recent study, Roig et al. [7] analyzed the systematic and periodical use, for 16 years, of anaerobically digested sewage sludge as an agricultural fertilizer by assessing the effects on some soil physical-chemical, functional, and ecotoxicological properties. They found that the input of sludge enhances soil properties proportionally to the application doses and/or frequency. In addition, the effects of organic matter, nitrogen, phosphorus and toxic elements in sewage sludge applied to agricultural land were reviewed by [8] and they found that the organic matter may improve the structure and water-holding capacity of poor soils and the nitrogen and phosphorus in sludge have fertiliser value, and the crops can accumulate toxic elements from sludge-amended soils.
In addition, Egiarte et al. [9] examined effect of repeated application of sewage sludge of short-term groundwater contamination and reported that a repeated application of sludge at 60 Mg∙ha−1 resulted in significantly higher concentrations of Zn, Cd, Cr and Ni in the leachates than with other treatments (other loading rates). Moreover, Wong et al. [10] investigated the effects of Dissolved Organic Matter (DOM) from anaerobically digested dewatered sludge on Cd and Zn sorption by three different soil types and found that the addition of DOM significantly reduced the Cd and Zn sorption capacity for tested soils, suggesting that DOM had a stronger inhibitory effect on Zn sorption than that of Cd. They also found that the reduction in metal sorption caused by DOM was very apparent in the pH range of 5 to 8, with a maximum inhibition on metal sorption occurring at pH 7 - 7.5 especially for Zn but the effect was minimal at lower pH.
Khan and Scullion [11] measured the effects of varying sludge metal (Cd, Cu, Ni, Pb and Zn) contents on respiration, biomass C and N, and N mineralization in a series of laboratory incubations of soil-sludge mixes. They found that Cd (up to 70 mg∙kg−1 in soil) did not affect any microbial index. Higher concentrations of the other metals generally caused a decrease in biomass C and N, the reduction for C often being proportionally less than that for N and in most cases, higher metal concentrations increased respiration rates and microbial metabolic quotient. In addition to that soil mineral N was increased by higher inputs of all metals and the use of sludges with higher metal concentrations may lead to short-term changes in soil microbial communities and their activities, with increased loss of C to the atmosphere and N availability.
Serna and Pomares [12] determined the N-mineralization rate of 12 sewage sludges in a given soil during a 16-week aerobic incubation by analysis of inorganic N produced by a nonleached procedure. They found that the aerobically treated sewage sludges gave higher mineralization rates than the anaerobically treated wastes and values of potentially mineralizable N (No) varied from 71 to 394 mg・N∙kg−1 soil, and mineralization rate constant (k) ranged from 0.089 to 0.883 week−1. In a different study, López-Valdez et al. [13] investigated how emissions of CO2, N2O and N2, and dynamics of mineral N were affected when different types of N fertilizer, i.e.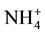 ,
, 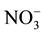 , or unsterilized or sterilized wastewater sludge, were added to the Texcoco soil. It was found that microorganisms added with the sludge accelerated organic material decomposition, increased
, or unsterilized or sterilized wastewater sludge, were added to the Texcoco soil. It was found that microorganisms added with the sludge accelerated organic material decomposition, increased  immobilization, and induced immobilization of
immobilization, and induced immobilization of 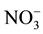 (in Texcoco soil). They suggested that wastewater sludge improves soil fertility at Otumba (an agricultural soil) and would favour the vegetation of the Texcoco soil (alkaline saline).
(in Texcoco soil). They suggested that wastewater sludge improves soil fertility at Otumba (an agricultural soil) and would favour the vegetation of the Texcoco soil (alkaline saline).
Fytianos and Charantoni [14] investigated the leaching of heavy metals from municipal sewage sludge. They found that for most values of liquid solid ratio (L/S), the percentage of leached amounts for the examined metals followed the order Cd > Zn > Pb> Fe > Mn. As pH decreased, metal concentrations measured in the leachate increased. And in general, EDTA showed the greatest mobilization ability, followed by NaOH, acid solutions (HCl, H3PO4), and water.
Moreno et al. [15] investigated the effect of sewage sludge amendment of a semiarid soil, previously polluted with Cd, on the toxic effect of this heavy metal on soil microbial biomass and its activity. They found out that in general, higher ED (Ecological Dose) values were calculated for the sewage sludge amended soil than for unamended soil and thus the Cd toxicity to microbial activity of the sewage sludge amended soil can be considered lower than that of the unamended soil. As obvious, very limited data on quantities of sludge are available however, no chemical analysis was done to determine concentrations of cations anions and heavy metal of sewage sludge from Gaza Strip. Accordingly, this study was designed to determine the cations, anions and heavy metals of sewage sludge from Gaza Strip and to test the possibility of using it as an alternative source of mineral fertilizers.
2. Materials and Methods
2.1. Sludge Sampling
A representative sludge samples with a volume of 20 L each were collected from different locations from the drying beds in the Sheikh Ejleen Waste Water Treatment Plant.
The samples were air-dried in the month of June 2013 using solar radiation, crushed and mixed together to insure homogeneity of the sludge. The dried sludge samples were sieved through 2 mm sieve and mixed again and kept in plastic bags for further experimental work.
2.2. Determination of Cations
Ten g of sludge samples were suspended in 25 ml distilled water form a ratio of 1:2.5 w/w and shaken over night, the pH and EC and TDS were measured to each sample. Then additional 25 ml water were added to form a ratio 1:5 w/w. Nutrient content (K, Ca, Mg, and Na, expressed as g∙kg−1 dry weight basis) were determined by EDTA titration (Ca, Mg), flame photometry (K, Na) [16] .
2.3. Determination of Anions
The sulfate (SO4) was determined using the turbidity method and in this procedure sulfate ion is converted to a barium sulfate suspension under controlled conditions. The resulting turbidity is determined by spectrophotometer at 420 nm and compared with a curve prepared from standard sulfate solution. While the orthophosphate was determined using ascorbic acid method. The chloride was determined using the titrimetric method by titrating it with silver nitrate (AgNO3) in the presence of potassium chromate as indicator
2.4. Determination of Heavy Metals
Following the procedure described by [17] , the heavy metal concentration in sludge samples will be described. In this procedure, 0.5 - 2 g air dried sludge were digested in 10 ml of nitric acid 78% and kept under heating with flux at 65˚C for 24 h. Then the system was heated up to 120˚C for another 24 h. After complete digestion (nearly clear solution appeared). The system was left for cooling at the room temperature. The digested sludge was filtered through Whattman scale 43, filter paper ashless. The collected filtrate was completed to the mark of volumetric flask capacity 25 ml with the same acid solution. Then the heavy metals were analyzed using Inductive Coupled Plasma analyzer (ICP).
2.5. Determination of Nitrogen Fractions
2.5.1. Nitrate Fraction
Nitrate level in the sludge was determined according to the salicylic acid method [18] which converts the nitrate concentration under acidic media to the corresponding nitrosalicylic acid with yellow color according to the chemical reactions shown in Figure 1(a).
The intensity of the yellow color represents the nitrate concentration and it was determined using a spectrophotometer. After making a standard curve of nitrosalicylic acid, the same procedure was done to the filtrate from 10 g of sludge in 50 ml of water. Results were expressed in g∙kg−1 dry weight basis.
2.5.2. Ammonium Fraction
Ammonium was determined using Kjeldahl method without the digestion step. Ten ml of the filtrate of 10 g of sludge in 50 ml of water after shaking overnight were taken to the distillation step of Kjeldahl and then to the back titration step to determine the ammonium fraction using hydrochloric acid. Results were expressed in g/kg dry weight basis.
2.5.3. Determination of Total Organic Nitrogen Fraction
Organic nitrogen (org-N) was determined using Kjeldahl digestion according to the following procedure below: 0.5 g of sludge samples were digested with sulfuric acid for 4 hours with 72% H2SO4 followed by a second boiling hydrolysis under reflux for 5 hours in H2SO4 (0.7 N) until clear solutions were obtained. Then the samples were transferred to the distillation unit at Kjeldahl apparatus to catch the produced ammonium in Boric acid. The ammonium borate was then titrated by HCl 0.1 N to calculate the N content in sludge samples. The reaction procedures are shown below. Details of chemical reactions are shown in Figure 1(b).
2.6. Statistical Analysis
The statistical analysis of data was performed with ANOVA test using Excel program. The samples were in three replicates; mean and standard deviation were calculated. Analysis of variance among treatments was performed using t-test, p-values below 0.05 indicate significant differences.
3. Results and Discussion
3.1. Determination of Sodium, Potassium, Calcium and Magnesium
Determination of Na and K were done using Flame photometer. The relationship between flame-photometer readings of Na+ and K+ concentrations are shown in Figure 2.
It is obvious that at low concentrations of Na or K ions, nearly linear relationship between the flame photometer read and the concentrations. However, fitting the data to a linear regression line showed bad fit (R2 = 0.55). Accordingly, converting the data in Figure 2 to a log scale provides good fit. The regression information is shown in Table 1. Where Y and X represent the log scales of flame photometer reading and concentrations respectively. Accordingly, the equations in Table 1 were used to determine the sodium and/or potassium concentrations in sludge samples. The measured concentrations are shown in Table 2.
![]()
![]() (a) (b)
(a) (b)
Figure 1. Conversion of nitrate to nitro-salcylic acid (a); Digestion of organic nitrogen containing compound (b).
![]()
Figure 2. Standard curve of (Na/K) determination in sludge sample.
![]()
Table 1. Regression equations for sodium and potassium.
![]()
Table 2. Sodium, potassium, calcium and magnesium concentrations in sludge samples.
Calcium and magnesium concentrations in the sludge samples were determined using EDTA titration method using plank and sample readings. The results are shown in Table 2.
It can be seen that Sodium and potassium concentrations are 28.93 ± 6.85, 2.53 ± 0.43 mg/kg, respectively. The high concentration of sodium or potassium ion in sludge samples may be attributed to the fact that a lot of surfactants, cosmetics, and soups are containing sodium or potassium in their chemical structure. Moreover, calcium and magnesium concentrations are 271 ± 38.74 and 177 ± 67 mg/kg, respectively. Furthermore, Fe concentration is 125.12 ± 13.65 mg/kg.
As obvious, K is the lowest concentration among all of the cations and Ca is the highest concentration among all cases.
The explanation of these results is that surfactants, soups, and cosmetics may be degraded during sludge formation resulting in transferring sodium or potassium into inorganic form (e.g. NaCl). Our explanation is supported by the results of [19] who found that surfactant, cosmetic product formulations, contributes significantly to the pollution profile of sewage and wastewater with various compounds including cations. Further supports to our results come from [20] who reported high concentration of cations in treated sewage water and from the results of [21] who correlated the high concentrations of cations in aerobic granular sludge with the low concentration of Cu++ in sludge.
3.2. Concentrations of Anions
Chloride concentration in sludge samples was determined according to the titration method using AgNO3 and NaCl as standard materials. The relationship between Cl concentrations and AgNO3 concentrations is shown in Figure 3. It can be seen that a linear regression equation provide the best fit (R2 = 0.9803). The high value of R2 suggests the best fit, accordingly the regression equation was used to determine the concentration of Cl− ions in the unknown samples. The determined Cl− concentrations in the unknown samples are shown in Table 3.
It is obvious that a linear relationship is shown in concentration at AgNO3 below 4 mg/l with an equation of Y = 0.2996X − 0.0106 with a value of R2 = 0.9803 indicating a strong positive association between the Cl (ml) and Na concentration mg/l. Accordingly, in the range of 0 - 4 mg/l, concentration of Cl may be determined. However, concentration above this range may need several dilutions. Nevertheless, within the range, a strong positive association was observed R2 = 0.9803. This relationship provides good fits that allow determination of Cl concentration in the sludge sample. However, the determined Cl concentration is 25.84 ± 4.26 mg/kg (Table 3) which is in the acceptable levels of Cl standards [22] .
3.2.1. Sulfate Concentration
The relationship between the optical density and sulfate concentration are linear below 60 mg/l as shown in Figure 4.
It can be seen that the relationship is expressed as a linear regression with equation Y = 0.035X + 0.066 and R2 value equals to 0.9971. This indicates a strong positive association and enabled accurate determination of sulfate concentration in the sludge samples. According, the determined sulfate concentration in sludge equals to
![]()
Figure 3. Relationship of standard solutions of Cl concentration and required amount of AgNO3.
![]()
Figure 4. Standard curve of sulfate concentration.
![]()
Table 3. Chloride, sulfate and phosphate concentrations. Values represent average and standard deviation.
18.59 ± 2.44 g/kg. This value looks nearly high but in fact sulfur containing protein is a high fraction in nature. However, the nearly high sulfate level is due to the high solubility in water which is not pH-dependent. Similar results were recently obtained by [23] .
3.2.2. Phosphate Concentration
The relationship between gradient concentrations of phosphates and corresponding optical densities are shown in Figure 5.
It can be seen that a linear relation is observed at concentrations below 10 ppm and the relationship is expressed by the equation Y = 0.3157X + 0.2313 with an R2 value equals to 0.9719. This indicates a strong positive association which enabled determination of phosphate concentrations in the sludge samples. The data are shown in Table 3.
A comparison among anions concentrations is shown in Table 3, it can be seen that Cl− has the lowest concentrations among all whereas ![]() is the highest among all.
is the highest among all.
The explanation of these results is that these values represent the soluble fraction of Cl−, ![]() , and
, and ![]() in sludge samples. The low value of phosphate concentrations in sludge samples is due to the fact that, phosphate solubility is pH-dependent. Accordingly, at low pH value high phosphate levels may be found in sludge. However, the current pH value of sludge equals to 6.78 ± 0.02 [24] accordingly, low fraction of phosphate was found. Another support to our argument comes from the fact that phosphoric acid is a weak acid and has 3 dissociation constants. This indicates that phosphate solubility is pH-dependent. It is obvious that the phosphate level is 0.434 ± 0.023 g/kg in the sludge. As obvious, nearly low values of
in sludge samples. The low value of phosphate concentrations in sludge samples is due to the fact that, phosphate solubility is pH-dependent. Accordingly, at low pH value high phosphate levels may be found in sludge. However, the current pH value of sludge equals to 6.78 ± 0.02 [24] accordingly, low fraction of phosphate was found. Another support to our argument comes from the fact that phosphoric acid is a weak acid and has 3 dissociation constants. This indicates that phosphate solubility is pH-dependent. It is obvious that the phosphate level is 0.434 ± 0.023 g/kg in the sludge. As obvious, nearly low values of ![]() are available in sludge samples. This value represent only the soluble fraction of phosphate not all phosphate. Under pH value 6.78, phosphate ion tends to precipitate from the solution. Similar observations were given recently by [25] .
are available in sludge samples. This value represent only the soluble fraction of phosphate not all phosphate. Under pH value 6.78, phosphate ion tends to precipitate from the solution. Similar observations were given recently by [25] .
3.2.3. Nitrate, Ammonium and TKN Concentrations
The importance of nitrate levels in sludge samples emerged from the fact that sludge can be used as an alternative source of nitrate instead of nitrogen, phosphorus and potassium fertilizer application in agriculture.
According to the methods described above, we generated a standard nitrate curve by converting the nitrate into the corresponding nitro-salicylic acid that has yellow color at alkali media and measured the optical density at 410 nm. Accordingly, the optical density of nitro-salicylic acid and its gradient concentrations are shown in Figure 6.
It is obvious that a linear relationship with a value of R2 = 0.9988 is obtained even at nearly high nitrate concentration (500 mg/l). This suggests that a strong positive association exists between the optical density and tested concentrations. This linear mode of interaction enabled us to use the regression equation to determine nitrate concentrations in the unknown samples of sludge. Accordingly, the linear relationship is expressed by the equation Y = 0.0008X. This equation was used to determine the nitrogen concentration in the sludge samples. The value of nitrate level in sludge equals to 871 ± 135 mg/kg sludge as shown in Table 4.
The nitrate level tends to be high. The explanation of this result is probably due to drying sludge, re-concen- tration of cations and anions may occur beside the fact that ammonium hydroxide may be converted to nitrate due to high O2 contents due to drying process. This explanation is supported by the results of [26] who investigated the nitrification performance in a membrane bioreactor treating industrial wastewater and concluded that mixing the municipal waste water with industrial waste water in the level up to 50% resulted in a breakdown of nitrification process. However, this level of nitrate is sufficient for plant nutrition. Furthermore, Total Kjeldahl Nitrogen (TKN) is nearly high and equals to 5000.04 ± 757.5 mg/kg sludge (Table 4). This high value presents all fractions of organic nitrogen. Our results agree with [27] who indicated that digested sewage sludge had high value of total nitrogen (2.17% ± 0.07%).
![]()
Figure 5. Standard curve of phosphate determination in sludge samples.
![]()
Figure 6. Optical density nitrate concentration relationship. Standard curve.
![]()
Table 4. Nitrogen levels in sludge. Values represent average and standard deviation.
The value of Total Kjeldahl Nitrogen (TKN) of the sludge is 5000.04 ± 757.5 mg/kg. The explanation of high TKN value is that sludge originated from human feces which contain a high fraction of protein due to high consumption of protein. It has been estimated that protein consumption per capita per day in Gaza Strip ranged between 20 - 40 g/capita/day. High fraction of this protein goes to the wastewater treatment plant and end up by sludge samples. Accordingly, high fraction of total Nitrogen was observed in sludge samples. Moreover, the total soluble nitrogen is 61.63 ± 31.69 mg/l, regardless of the high value of standard deviation; the total soluble nitrogen is nearly in the expected range. This includes ammonia, amines and amino-acids. Recent papers showed similar results [28] . This value of total soluble N is considered moderate level. However, the explanation of this result is that all nitrogen fractions in wastewater or sludge are present in the form of organic nitrogen or in the reduced form (NH3, NH4OH) due to the anaerobic condition of TWW or sludge. Accordingly, moderate to low values of soluble N may be found. Our results are also supported by the previous work of [29] who found low nitrate concentration in the treated wastewater.
3.3. Concentration of Heavy Metals
Concentration of heavy metals in sludge samples are shown in Table 5. It can be seen that cupper concentration is 172.56 ± 61.53 mg/kg. Moreover, lead concentration is 75.86 ± 1.63 mg/kg. Furthermore, the zinc concentration is 218.73 ± 8.35 mg/kg. The manganese concentration is 157.96 ± 21.67 mg/kg. It is obvious that Zn concentration is the highest one among all cases followed by cupper and manganese. Moreover, lead concentration is the lowest one among all.
The high levels of heavy metals can be explained by the fact that industrial wastewater network is connected with the domestic wastewater network. Both wastewaters come together to wastewater treatment plant. The mixing of industrial wastewater with domestic wastewater allows heavy metals to precipitate due to the changes of pH values from acidic range in the industrial wastewater to alkaline range in the domestic wastewater. The precipitated heavy metals react with organic acid that generated from the biodegradation of the organic wastes in the treatment plant and with inorganic acid.
This process makes an accumulation of heavy metals in sludge. Our explanation is supported by the results of [30] who investigated the distribution and variation trend of heavy metals in sludge samples collected from different wastewater treatment plants in China and showed that contents of heavy metals in sludge varied significantly, and the average contents exhibited an order of Cr > Zn > Cu >Pb> As > Hg > Cd. However, the presence of heavy metal in sludge sample may move to the plant during the agricultural process [29] . Furthermore, the presence of heavy metals in sludge may become toxic to soil nitrifying or denitrifying bacteria that benefit the plant growth. This explanation is supported by the results of [31] who studied the effects of heavy metals in wastewater on anoxic/aerobic-membrane bioreactors performance and revealed that the nitrification/denitrifica- tion rates were dramatically decreased in different percentages based on the concentration of heavy metals in wastewater. Accordingly, it may be necessary and recommended to remove heavy metals from sludge samples before agricultural application. Our recommendation is supported by the work of [32] who investigated the long-term removal of heavy metals in a combined up-flow anaerobic sludge bed system treating municipal wastewater and revealed that high removal efficiencies were found for some metals in the following order: Sn > Cr > Cu > Pb > Zn > Fe (63% - 94%) and medium removal efficiencies for Ni (49%), Hg (42%), and Ag (40%), and finally Mn and As showed negative percentage removals. Further support come from the results of [33] who studied the retention of heavy metals at two pilot-scale treatment wetlands, consisting of two vertical flow beds followed by a horizontal flow bed and revealed that a major removal pathway was sedimentation and adsorption onto soil substrate as well as precipitation and co-precipitation. It has been shown by [34] that application of compost containing high fraction of heavy metals expose plant and ground water to contamination of heavy metals. In addition, El-Nahhal [20] found heavy metals in the drinking water collected from different sources and attributed this to possible contamination from industrial wastewater out house. In comparison with USEPA 1994 standards, the heavy metals concentrations in the sludge are found to be many times lower than USEPA 1994 standards. Accordingly, the sludge can be used in agriculture.
4. Conclusion
Sludge samples contain high fraction of Na, K, Ca, Mg, and Fe which are in respective manner 28.93 ± 6.85, 2.53 ± 0.43, 271 ± 38.74, 177 ± 67 and 125.12 ± 13.65 mg/kg. Moreover, ![]() ,
, ![]() , Cl− are respectively 25.84 ± 4.26 mg/kg, 18.59 ± 2.44 g/kg and 0.434 ± 0.023 g/kg. Sludge samples contained high fraction of
, Cl− are respectively 25.84 ± 4.26 mg/kg, 18.59 ± 2.44 g/kg and 0.434 ± 0.023 g/kg. Sludge samples contained high fraction of
![]()
Table 5. Heavy metals levels in sludge. Values represent average and standard deviation (mg/kg).
NA = not available.
TKN. Total soluble N and nitrate are in respective form: 5000.04 ± 757.5, 61.63 ± 31.69 and 871 ± 135 mg/kg. The concentrations of Cu, Pb, Zn, Mn are in the following order: 172.56 ± 61.53, 75.86 ± 1.63, 218.73 ± 8.35 and 157.96 ± 21.67 mg/kg, respectively. Application of sludge in agriculture soil may enhance plant growth due to high fraction of plant nutrients.