1. Introduction
Phytoremediation takes advantage of the unique, selective and naturally occurring uptake capabilities of plant root systems, together with the translocation, bioaccumulation and pollutant storage/degradation abilities of the entire plant body. Other advantages include the economy of the process which, on an average is about ten-fold cheaper than other physical, chemical or thermal remediation methods since it is performed in situ, is solar driven and can function with minimal maintenance once established [1] [2] . An added advantage is that the process can serve to be recreational and be aesthetically pleasing at the same time.
In India, at present, there are many leather manufacturing industries (tanneries) located in the states of Uttar Pradesh and Punjab. The raw material for these tanneries is cow/buffalo hide and goat/sheep skins. In the tanning industry, about 25% of the weight of raw hides’ results in finished leather whereas the remaining 75% becomes a solid waste. Sludge is a mixture of solid wastes and bacteria, removed from the wastewater at various stages of the leather preparation process. The conventional chemical processes in leather tanning industries are often restricted because of technical or economical constraints and generate large amount of toxic sludge. The effluent from such industries is subjected to physico-chemical treatment such as screening, grit removal, equalization, chemical coagulant addition, flocculation and sedimentation before being subjected to two-stage activated sludge treatment that generates primary and secondary sludge. The effluent of the activated sludge treatment is subjected to tertiary treatment in the form of coagulation and sedimentation. Sludge, in general, represents a stress condition for growth of the plants and can induce conditions of oxidative stress [3] [4] .
Presently, there is no proper sludge disposal system in tanneries in India. The dried sludge is removed from the sludge drying beds and disposed of in the tannery surroundings indiscriminately, without any environmental consideration. This method of sludge disposal has so far been considered as a low-cost solution for disposal of hazardous wastes.
Chromium is a well-known highly toxic heavy metal considered as a priority pollutant. It is a nonessential metallic element belonging to the first transitional series of the periodic table and is of particular concern to surface water and soil pollution. Wastewaters from industries like leather tanning, electroplating, paint and pigments, dying, canning, textile and production of steel contain large amounts of chromium. Normally industrial wastewaters contain both Cr (VI) and Cr (III) ions at concentration ranging from 10 to 100 mg/L [5] . Accumulation of Cr (III) ions can inhibit various enzyme systems of living organisms and also affect the ecological environment when present in large amounts. The presence of chromium in aquatic ecosystem poses human health risks and causes harmful effect to living organisms [6] .
Under natural conditions of growth and development, plants are inevitably exposed to different types of stress, which may cause increased production of reactive oxygen species (ROS) [7] . These include super oxide radicals ( ), singlet oxygen (1O2), hydrogen peroxide (H2O2) and hydroxyl radical (
), singlet oxygen (1O2), hydrogen peroxide (H2O2) and hydroxyl radical ( ), which cause tissue injury [8] [9] . Plants have evolved various protective mechanisms to eliminate or reduce ROS. In plant cells, one of the protection mechanisms is antioxidant system, composed of non-enzymatic and enzymatic antioxidants [8] . The capacity of the antioxidant defense system is often increased under stress condition [10] , but in most situations the response is moderate.
), which cause tissue injury [8] [9] . Plants have evolved various protective mechanisms to eliminate or reduce ROS. In plant cells, one of the protection mechanisms is antioxidant system, composed of non-enzymatic and enzymatic antioxidants [8] . The capacity of the antioxidant defense system is often increased under stress condition [10] , but in most situations the response is moderate.
These ROS are detoxified by the sequential and simultaneous action of a number of enzymes including glutathione reductase (GR), superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) and glutathione-S-transferase (GST). GSTs have direct cytoprotective activities and they might be essential for the preservation of plants during environmental stress and disease, as well as for the support of normal development [11] . In addition to catalyzing GSH conjugation, GSTs also exhibit glutathione peroxidase (GSH-POX) activity, which suggests a role in protection against oxidative stress.
There is currently no report on bioaccumulation potential of Catharanthus roseus (family Apocynaceae) for Cr from low level waste. The main objective of this research was to evaluate the phytoremediation potential of C. roseus and study the effect of Cr stress on growth characteristics and alkaloid content of C. roseus. Recent studies have indicated that heavy metal stress increases the activity of antioxidant enzymes which play important roles in adaptation of plants to stress conditions [12] . Consequently, one of the aims of this study was also to examine whether Cr induced stress affects the antioxidant defense system, as well as the accumulation of alkaloids in C. roseus. The genus Catharanthus has gained considerable reputation in the therapeutic world for its wide assemblage of over 100 alkaloids including vincristin, vinblastin, ajmaline, ajmalicine and serpentine which are extremely important [13] [14] . The plant, though can be cultivated in gardens as a flowering plant, is known for its robust growth in wastelands and is toxic due to the presence of toxic alkaloids. Pandey et al. [15] have studied the impact of cadmium and lead on C. roseus. Zheng and Wu [16] have reported that cadmium treatment enhanced the production of alkaloid and secondary metabolites in C. roseus. Since stress condition provided suitable environment for synthesis and accumulation of secondary metabolites, C. roseus was chosen to study its phytoremediation potential with respect to alkaloid production. In the present investigation, the phytoremediation potentials of C. roseus as evinced by its bioaccumulation coefficient (BAC) with respect to chromium, as well as the status and developmental activity profiles of enzymes POD and GST in leaves of plants grown in sludge amended soil were determined and evaluated.
2. Materials and Methods
2.1. Materials
All chemicals used in enzyme assays were purchased from Sigma Chemical Co. St. Louis (USA). 1-chloro-2, 4- dinitrobenzene (CDNB) was from Spectrochem Pvt. Ltd. Mumbai, India. All other chemicals used were of analytical grade.
2.2. Seed Collection and Sampling
Seeds of C. roseus var. rosea were collected from the experimental gardens of Department of Life Sciences, ITM University, and were dried under the shade. Seeds were stored in screw-cap vials at 10˚C. Seeds were soaked overnight in distilled water. C. roseus seeds were subsequently washed with 1% HgCl2 for 5 min to remove any fungal contamination. After several rinses in distilled water, the seeds were germinated in garden-beds of soil to a depth of 1 cm under natural conditions.
2.3. Physico-Chemical Analysis of Sludge and Garden Soil
Physico-chemical parameters viz. pH, moisture content and settable solids (SS) of primary and secondary sludge as well as garden soil were determined.
2.4. Preparation of Pots for Growth of C. roseus Plants
One month old seedlings of C. roseus were chosen for experimental purpose. Garden soil was left overnight to lessen the moisture content. The dried soil was ground and sieved. Subsequently, pots for growth of C. roseus seedlings were prepared. Each pot contained a 500 g mixture of soil and primary/secondary sludge in different concentrations obtained from Leather Technology Park, Banthar, Unnao, India. A total of 3 sets of experiments were set up each containing 7 pots. The first pot of each set contained seedling grown under normal conditions (0% sludge). This plant was treated as the control. The soil in experimental pots was amended with different percentage of primary and secondary sludge. The second, third and fourth pot contained primary sludge at percentage of 10%, 20% and 50%, respectively. Similarly, the fifth, sixth and seven pots contained secondary sludge at percentage of 10%, 20% and 50%, respectively. After the plantation of seedlings, all pots were kept in sunlight for 28 days for growth observation. Morphological changes were observed thereafter.
2.5. Plant Harvest and Analysis
Plants from each of the 21 pots (comprising three sets of experiments) were gently removed from the pots after 4 weeks (28 days) for assessment of various growth parameters and biochemical analysis. Shoots and roots from each plant were separated and washed with distilled water for 20 min and divided into separate bundles. Leaves were plucked from the apical regions of the shoots and washed properly with tap water followed by distilled water. The leaves were dried with the help of Whatman filter paper and weighed. The dried leaves were divided into two groups. The leaves of one group were then homogenized in a pestle-mortar in minimal amount of 0.1 M Tris-HCl buffer, pH 7.5 and centrifuged at 10,000 rpm for 20 min. The supernatant was subjected to analysis of chromium content. The roots were subjected to a similar treatment for analysis of chromium uptake and content. The antioxidant enzymes and total alkaloid content was estimated in the other group of roots and shoots.
2.6. Estimation of Settable Solids (SS)
One liter samples of primary/secondary sludge and/or garden soil were taken in a measuring cylinder and filtered through a weighed standard glass-fiber filter and the residue retained on the filter was dried to a constant weight at 104˚C. The increase in weight represented the total suspended solids (TSS) which were estimated using the formula:

where A = weight of filter + dried residue; B = weight of filter (mg).
For estimation of SS, 1 L samples were allowed to stand quiescent for an hour. Without disturbing the settled or floating material, 250 mL was siphoned from center of measuring cylinder at a point halfway between the surface of the settled material and the liquid surface. TSS (mg/L) was determined in this supernatant liquor as mentioned above. These were the non-settable solids. The SS were estimated using the formula:
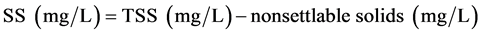
2.7. Chromium (VI) Analysis
To analyze chromium content, any trivalent chromium present in primary/secondary sludge and leaf/root homogenate was first converted into the hexavalent state by oxidation with potassium permanganate at a high temperature (130˚C - 140˚C) under acidic conditions. Thereafter, the chromium concentration was determined by colorimeter using the diphenylcarbazide (DPC) detection method according to Bartlett [17] . This method is a slight modification of the procedure published in Standard Methods for the Examination of Water and Wastewater, 20th ed., American Public Health Association. DPC solution was prepared (0.25% w/v in 50% acetone). 15 mL each of the oxidized soil solution/leaf/root homogenate, containing Cr (VI) were pipetted out into 25 mL standard volumetric flasks. To these, 2.0 mL of 3 N H2SO4 was added followed by 1.0 mL of DPC and the total volume in each tube was made up to 25 mL using distilled water. The absorbance of the resulting red-violet sample was measured against a reagent blank at 540 nm using a spectrophotometer.
2.8. Calculation of Bioaccumulation Coefficient (BAC)
The following formula was used for calculation of BAC = Element concentration in plant part (µg metal per g dry weight of plant part)/Element concentration in soil (µg metal per g dry weight of soil) [18] .
2.9. Preparation of POD and GST from Leaves of C. roseus
C. roseus leaves were homogenized in a pestle-mortar at 4˚C in minimal amount of 0.1 M Tris-HCl buffer, pH 7.5. The solution was then centrifuged in a pre-cooled centrifuge at 10,000 rpm at 4˚C for 20 min. Supernatant was taken and stored for further use as source of enzymes POD and GST.
2.10. Salt Fractionation of C. roseus Leaves Homogenate to Partial Purification of POD and GST
For enrichment in enzyme activity, homogenate of C. roseus leaves was subjected to 0% - 80% ammonium sulfate fractionation [19] . After each fractionation, the sample was stirred in cold for 30 min and then centrifuged at 13,000 g for 15 min. All the fractions were analyzed for enrichment in POD and GST activity. The fractions having highest enrichment in POD and GST activity were used as source of POD and GST.
POD activity determination: POD activity was assayed in 0.025 mL aliquots of ammonium sulfate saturated fractions of crude leaf homogenate as described by Putter [20] , with slight modification. Peroxidase activity in crude homogenate of leaves of C. roseus was determined through colorimeter at 470 nm with substrate H2O2 and dye o-dianisidine (DAS). The oxidation of the reduced form of dianisidine (molar extinction coefficient 11.3 mM−1∙cm−1) produced a brick red color readable at 470 nm in a colorimeter maintained at 37˚C. The assay mixture contained 1.0 mL of 0.1 M phosphate buffer, pH = 6.5, 0.5 mL of 0.2 M H2O2, 0.2 mL of 0.01 M o-dianisidine (DAS), 0.275 mL of distilled water and 0.025 mL of crude supernatant as a source of enzyme. The assay mixture was taken in a cuvette and the absorbance was taken at a time interval of 30 sec for 5 min in a colorimeter. Enzyme activity was expressed as μmol oxidized dye formed/min ± S.D. based on experiments done in triplicates.
GST activity determination: GST activity was assayed spectrophotometer at 340 nm in 0.025 mL aliquots of ammonium sulfate saturated fractions of crude leaf homogenate according to the method of Habig et al. [21] . The reaction mixture contained 100 mM phosphate buffer, pH 6.5, 1.0 mM CDNB in 20 μl ethanol, 1.0 mM GSH and enzyme protein. Enzyme activity was expressed as μmol S-2, 4-dinitrophenyl-GSH adduct formed/min ± S.D., using a molar extinction coefficient of 9.6 mM−1∙cm−1 for CDNB.
2.11. Activity Staining
Native PAGE was performed on a 7.5% gel according to the method of Laemmli [22] . Gels were stained for peroxidase and GST activity after native PAGE. For peroxidase staining, gels were incubated for 5 - 10 min at 30˚C in a reaction mixture comprising 10.0 mL 0.1 M potassium phosphate buffer pH = 7.0, 20 mg Benzidine (which was dissolved in methanol) and 0.2% H2O2 After incubation the gel was rinsed in distilled water and kept for observation. The sites on the gel where the enzyme peroxidase was present were stained blue which after sometime turned brown confirming the presence of native peroxidase.
Gels were stained for GST activity after native PAGE using the method of Ricci et al. [23] . Blue insoluble formazan appeared on the gel surface in about 3 - 5 min, except in the GST area.
2.12. Protein Estimation
Protein was estimated in crude homogenate/ammonium sulfate fractions using BSA as standard [24] .
2.13. Extraction and Estimation of Total Alkaloids from Roots and Leaves of C. roseus
Total extraction and determination of alkaloids from C. roseus roots and leaves was carried out on day 28 as described by Endo et al. [25] with slight modification. Samples of leaves and roots were homogenized in 90% ethyl alcohol. The ethanol extract was evaporated to dryness. The residue was dissolved in distilled water and mixed with conc. HCl (final concentration of HCl was 3%). An equal volume of ethyl acetate was added. The aqueous phase was collected and adjusted to pH 9.0 with ammonia and extracted with chloroform. The chloroform phase containing the alkaloids was collected and evaporated to dryness to get total alkaloids. The alkaloids were detected at 254 nm. Standard curve was prepared with a mixture of ajmalicine and ajmaline.
2.14. Statistical Analysis
In all experiments three replicates were done for each concentration of primary and secondary sludge. Mean and standard deviations were calculated from triplicate measurement of three separate experiments. For differences between seven mean values (one control and six treatment groups), an analysis of variance (ANOVA) was performed. Results were considered to have reached statistical significance when p < 0.05. Critical difference was calculated to compare between various treatments. Significant differences of the means were set at p < 0.05.
3. Results and Discussion
3.1. Effect of Different Concentrations of Cr Containing Sludge Amended Soil on Plant Growth
Primary and secondary sludge were analyzed for their physico-chemical properties (Table 1). Figure 1 depicts the amount of Cr in leaves and roots of C. roseus plants when they were grown in presence of 10% - 50% primary and secondary sludge.
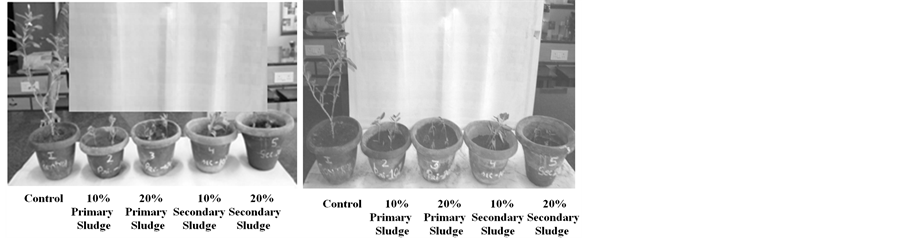
Considerable Cr uptake was observed in the roots of treated plants as compared to control (Figure 1). The plants grown in 10% and 20% primary sludge showed a significant decrease in growth as compared to control (Figure 2). The leaves of C. roseus plants showed signs of senescence when grown in 20% primary sludge amended soil. In plants grown in 50% primary sludge amended soil, chlorosis and senescence occurred (Figure 3). C. roseus plants grown in 10%, 20% secondary sludge amended soil also showed a significant decrease in growth as compared to the control. C. roseus plants showed a good amount of Cr accumulation in the roots and leaves as estimated by colorimeter. The data showed that periwinkle could absorb up to about 38% of the

Figure 1. Chromium concentration in control and treatment groups (µG/G).
 (a) (b)
(a) (b)
Figure 2. Effect of Cr concentration on plants grown in 10% and 20% primary/secondary sludge amended soil after (a) 2 weeks and (b) 4 weeks.
 (a) (b)
(a) (b)
Figure 3. Effect of Cr concentration on plants grown in 50% primary/ secondary sludge amended soil after (a) 2 weeks and (b) 4 weeks. (From left to right) Pots 1, 2-primary sludge, & pots 3, 4-secondary sludge.

Table 1. Physico-chemical properties of primary/secondary sludge and garden soil. Values are mean ± S.D. based on experiments done in triplicates.
amount of Cr present in primary and secondary sludge amended soil through roots and accumulate it to about 22% in leaves.
3.2. Effect of Different Percentage of Primary Sludge Amended Soil on the Developmental Profile of POD and GST from Leaves of C. roseus
Figure 4(a) and Figure 4(b) respectively show the status of POD and GST activities in ammonium sulfate precipitated P55 and P75 fractions from leaves of control plants vs. treated plants. POD activity was found to be localized mainly in the P75 fraction whereas GST activity was found to be more or less equally distributed between both fractions. There was a significant increase in POD activity in the plant grown in 10% sludge amended soil. At 20% sludge there was a decrease in the enzyme activity and at 50% sludge no enzyme activity was detectable in the leaves due to plant senescence (Figure 4(a)). A similar trend was observed in the pattern of GST activity from leaves of C. roseus (Figure 4(b)). On the other hand, plants grown in 10% and 20% secondary sludge amended soil showed no significant alteration in the POX as well as GST enzyme activity in their leaves. However, at 50% secondary sludge augmentation, no appreciable activity was detected.
3.3. Activity Staining
Figure 5(a) and Figure 5(b) depict the significant and detectable increase in expression of enzymes peroxidase and glutathione-S-transferase in leaves of plants grown in 10% sludge amended soil as compared to control plants. Interestingly, two bands were observed in case of peroxidase staining, thereby indicating the possible
 (a)
(a) (b)
(b)
Figure 4. (a) Status of POD and (b) GST in from leaves of plants grown in soil amended with 10% - 50% of primary and secondary sludge after 4 weeks. (Values are mean ± S.D. of 3 separate experiments. p < 0.05 with respect to control).

Figure 5. Activity staining of partially purified peroxidase (a) and glutathione-S-transferase (b) from leaves of control and 10% sludge treated C. roseus plants.
expression of a new isozyme under stress conditions in these plants. A crucial step in the synthesis of vincristine and vinblastine is the coupling of catharanthine and vindoline to produce the dimeric precursor α-3’,4’-anhydrovinblastine (AVLB). This new isozyme might be a putative peroxidase bearing an AVLB synthase activity.
3.4. Effect of Cr on Accumulation of Alkaloids in Roots and Leaves of C. roseus Plants Grown in Primary and Secondary Sludge Amended Soil
The total indole alkaloid accumulation significantly increased in presence of primary sludge in comparison to control. The alkaloid content in roots and leaves was found to be maximal in C. roseus plants grown in 20% primary sludge amended soil (Figure 6). On the other hand, a non significant increase in the alkaloids content was observed in the roots and leaves of plants grown at various concentrations of secondary sludge amended soil.
Phytoremediation is an emerging eco-friendly, cost effective, in situ treatment technology [26] . Heavy metals have become one of the main biotic stress agents for living organisms because of their increasing use in the developing field of industry causing high bioaccumulation and toxicity [27] . Heavy metal toxicity usually depends on the metal amounts accumulated by plants [28] .
Among the phytoremediation methods, phytoextraction is considered the best approach to remove the contamination primarily from soil without destroying the soil structure and fertility [29] . It is also referred as phytoaccumulation. As the plants absorb, concentrate and precipitate toxic metals from contaminated soils into the biomass, it is best suited for the remediation of diffusely polluted areas, where pollutants occur only at relatively low concentration and superficially [30] . Discovery of hyperaccumulator species has further boosted this technology. In the natural setting, certain plants have been identified which have the potential to uptake heavy metals. At least 45 families have been identified to have hyperaccumulator plants; some of the families are Brassicaceae, Fabaceae, Euphorbiaceae, Asteraceae, Lamiaceae, and Scrophulariaceae [31] [32] . Among the bestknown hyperaccumulators is Thlaspicaerulescens commonly known as alpine pennycress [33] , without showing injury it has been shown to accumulate up to 26,000 mg∙kg−1 Zn; and up to 22% of soil exchangeable Cd from contaminated sites [34] [35] . Brassica juncea, commonly called Indian mustard, has been found to have a good ability to transport lead from the roots to the shoots [36] .
So far, no study has been reported about the phytoremediation efficiency and phytoaccumulation potential of C. roseus with respect to chromium. In the present study, the effect of chromium on the activities of antioxidant enzymes viz. POD and GST in leaves and alkaloid accumulation in leaves and roots of Catharanthus roseus was investigated. The plant C. roseus was found to be resistant to heavy metal chromium contamination in soil. C. roseus plants showed tolerance to Cr concentration up to 375 μg/g soil. The plants showed senescence when grown in 50% primary sludge amended soil (Cr concentration 937.5 μg/g). It must be noted that heavy chromium contamination/concentration was found to be growth inhibitory, as it decreased biomass in all respects and finally decreased total alkaloid content also. Periwinkle was shown to absorb up to about 38% of the amount of Cr present in primary and secondary sludge amended soil through roots and accumulate it to about 22% in
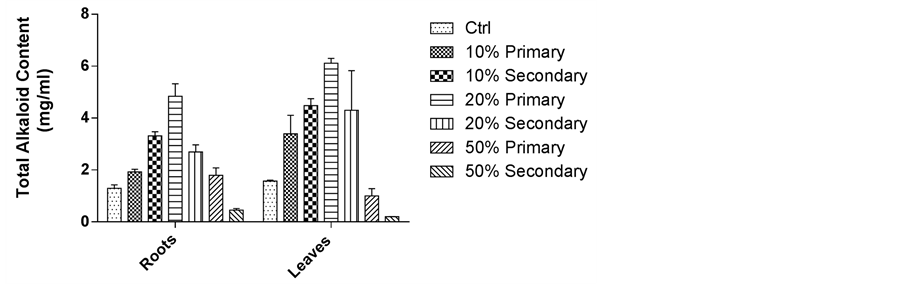
Figure 6. Effect of chromium on total alkaloid content in roots and leaves of C. roseus plants grown in soil amended with 10% - 50% of primary and secondary sludge after 4 weeks. (Values are mean ± S.D. of 3 separate experiments. p < 0.05 with respect to control).
leaves. The results obtained are concurrent with the findings of Pandey et al. [15] . The growth of C. roseus significantly decreased in presence of sludge as compared to that of control in soil (Figure 2(a) and Figure 2(b)). With the increase in plant growth in presence of 10% primary and secondary tannery sludge, the activities of antioxidant enzymes POX and GST increased significantly in leaves. It is known that under stress condition plants generally shift a major portion of their metabolic activities towards secondary metabolite synthesis, so an increase in alkaloid content was expected [13] [15] . A simultaneous increase in the alkaloid content of roots and leaves was observed (10% - 20% primary sludge).
In the present investigation, seeds of C. roseus is were grown the plants in garden soil devoid of any heavy metal for one month and subsequently one-month old seedlings were then transplanted into pots containing sludge amended soil to study Cr uptake and bioaccumulation. Further studies would involve studies focusing on the effect of Cr on seed germination. Also, the effect of Cr on various growth parameters like plant height, root/ shoot length, number of branches and flowers, peduncle length, head diameter, fresh and dry flower weight as well as effect on chlorophyll content, fertility etc., would be evaluated. Furthermore, the relationship between Cr and mechanism of ROS generation would be investigated.
4. Conclusion
Chromium causes oxidative stress as evidenced by increased alkaloid accumulation. Moreover, the data demonstrated a significant increment in the activities of two major enzymes, which are involved in the scavenging and detoxification of ROS. It further remains to be investigated whether this increase in the activities of the respective enzymes is due to induced gene transcriptional and de novo synthesis of proteins, or due to posttranslational modification of existing protein. The potential of C. roseus to take up Cr from the soil has been established by screening the plants and studying the phytoremediation of Cr at various concentrations by an eco-friendly, solarenergy driven in situ remediation technology that utilizes the inherent ability of living plants to clean up the environment. Our experimental data demonstrated that C. roseus can well tolerate low amounts of chromium (and accumulate it to about 22% in leaves) and can, thus, be grown on tannery sludge contaminated land and/or sites with low chromium contamination, where it might prove useful in the reclamation and remediation of chromium contaminated soil and land.
Acknowledgements
Authors are thankful to Dr. R.K. Pandey, Vice Chancellor, ITM University, Gwalior, MP, and College of Natural and Applies Sciences (CONAS) Crescent University, Abeokuta, Nigeria for their support and encouragement.
NOTES
*Corresponding author.