Comparison of transient and stable expression of foot-and-mouth disease virus capsid proteins in mammalian cells ()
1. INTRODUCTION
Foot-and-mouth disease virus (FMDV) is a highly contagious virus of cloven-hoofed animals, which belongs to the Picornaviridae family [1]. Its genome encodes a polyprotein that is processed by virus-encoded proteases into structural and non-structural proteins. The viral capsid precursor P12A is cleaved by protease 3C into the capsid proteins VP0, VP3 and VP1, which spontaneously self-assemble into different subviral particles. One copy of VP0, VP3 and VP1 forms the protomer (5S), five protomers assemble into the pentamer (12S) and twelve pentamers form the empty capsid (75S) [2]. Cleavage of VP0 into VP2 and VP4 occurs upon encapsidation of genomic RNA.
Nowadays, vaccination is the main means of foot-andmouth disease (FMD) control in most endemic areas. The vaccine is produced by growing FMDV in BHK-21 cell cultures under biosecured conditions [3]. Although this vaccine has proved to be effective in preventing the disease, its production entails a number of disadvantages, including the risk of virus escape, the problem of discriminating between vaccinated and infected animals, and difficulties of certain serotypes and subtypes to grow well in cell cultures [4]. To overcome these problems, new approaches intend to find alternatives that could provide the immunogenicity of the current vaccine while avoiding the handling of large quantities of infectious virus [5]. Among these, empty capsids appear to provide an excellent subunit vaccine candidate because FMDV empty capsids are naturally produced in infected cells and are as immunogenic as virions. Many approaches for the production of FMDV empty capsids have mainly focused on the use of the baculovirus expression system and recently the use of recombinant vaccinia virus [6-8]. However, the use of nonviral vectors in mammalian cells has been poorly explored. Mammalian cell expression system can be used for transient or stable expression depending upon the purpose and the properties of the recombinant protein. For stable expression the gene of interest must integrate into the genome of the host cell.
Although the process is tedious and time consuming, once the cell line is established it represents an unlimited source of protein. For transient expression, after transfection with a recombinant plasmid, production of the recombinant protein will occur until the cells are harvested. The gene of interest is not integrated into the genome of the host cells. Transient expression is an alternative and feasible approach for the production of toxic proteins that impairs the isolation if stable clones [9]. The production of recombinant FMDV empty capsids requires the expression of the poliprotein P12A and protease 3C. Protease 3C has been reported to cleave not only the FMDV capsid precursor but also histones and initiation transcription factors of the host cell [10,11]. Thus, since protease 3C can affect protein expression it can affect the isolation of high expressing clones. In order to determine the best strategy for the production of FMDV empty capsids, we compared transient and stable expression in BHK-21 cells using a recombinant plasmid.
2. MATERIALS AND METHODS
2.1. Cells and Viruses
Baby hamster kidney cell (BHK-21) monolayers were grown in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% fetal bovine serum (FBS) in a humidified incubator at 37˚C with 5% CO2. FMDV serotype A/Arg/01 inactivated with binary ethyleneimine and purified by sucrose gradient was used as a positive control for protein characterization assays.
2.2. Plasmids
Gene fragments encoding for the capsid precursor P12A (2258 bp) and protease 3C (687 bp) were amplified by PCR from F3A and F3B plasmids (kindly provided by Dr. Soledad García Nuñez, Instituto de Biotecnología, INTA, Argentina), respectively. F3A and F3B plasmids encoded the complete sequence of FMDV A/Arg/01. Amplicons were cloned into pGEMT-Easy vector (Promega) and subcloned together into the pCI-neo mammalian expression vector (Promega). The protease 3C was also cloned alone. Plasmids were named pCI-P12A3C and pCI-3C, respectively. The primers used for amplification of P12A and 3C for the generation of pCI-P12A3C were: P12A foward, 5’-GCT AGC GCC GCC ACC ATG GGG GCT GGA CAA TCC-3’, P12A reverse, 5’-TTC GAA CCC AGG GTT GGA-3’ and 3C foward, 5’-TTC GAA AGT GGT GCC CCA CCG-3’, 3C reverse, 5’-CTC GAG TTA CAT CAC GTG AAC GCG-3’. The primers used for amplification of 3C for the generation of pCI-3C were: 3C foward, 5’-CTC GAG AAC ATG AGT GGT GCC CCA CCG-3’, 3C reverse, 5’-GCT AGC TTA CAT CAC GTG GAC GCG-3’. Restriction enzyme sites are indicated underlined.
2.3. Protein Expression in BHK-21 Cells
For transient expression, BHK-21 cells were transfected with pCI-P12A3C plasmid in 25 cm2 T-flasks with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Twenty-four hours post-transfection, cells were treated with 1.5 ml of lysis buffer (20 mM TrisHCl, 137 mM NaCl, 10% (v/v) glycerol, 1% (v/v) nonidet P-40, 2 mM EDTA) and analyzed for recombinant protein expression. For stable cell line generation, BHK-21 cells were transfected with BamHI-linearized pCI-P12A3C and selected in the presence of 700 µg/ml geneticin (G-418 sulfate, Gibco-BRL). After 2 weeks of selection, surviving cells were subjected to terminal dilution and emerging clones were tested for recombinant protein expression.
2.4. Evaluation of the Effect of Protease 3C on Protein Expression
BHK-21 cells were co-transfected with pcDNA-EGFP (kindly provided by Dr. Gabriela Calamante, Instituto de Biotecnología, INTA, Argentina) and either empty pCIneo vector or pCI-3C in 24 well culture plates with Lipofectamine 2000. After 48 hours, cells were observed by fluorescent microscopy and analyzed by flow cytometry using a FACSCalibur (BD biosciences). In order to corroborate that the effect observed was due to the activity of protease 3C and not to the presence of the recombinant plasmid, pCI-3C was linearized with SalI (which cleaves inside the 3C gene) or with BstBI (that cleaves the vector backbone downstream the stop codon/ A tailing). Both vectors were named pCI-3Ci (inactive) and pCI-3Ca (active), respectively and were co-transfected with pcDNA-EGFP.
2.5. Characterization of FMDV Expressed Proteins
Cell lysates were separated by SDS-PAGE. Separated proteins were transferred onto a nitrocellulose membrane, blocked and then incubated with anti-FMDV guinea pig serum. After several washes, membranes were incubated with horseradish peroxidase-conjugated antiguinea pig goat serum (KPL). The reaction was visualized with an enhanced chemiluminescence method. Quantification of recombinant proteins was carried out by enzyme-linked immunosorbent assay (ELISA). Briefly, microtiter plates (Immunolon II) were coated with anti-FMDV rabbit serum (1/3000) in carbonate-bicarbonate buffer, pH 9.6, at 4˚C overnight. After washing and blocking, cell lysates were added and incubated on plates at 37˚C for an hour. Known amounts of purified inactivated FMDV were two-fold serially diluted and added to the wells for standard curve generation. Plates were then incubated with anti-FMDV guinea pig serum, followed by horseradish peroxidase-conjugated antiguinea pig goat serum (KPL). O-phenylenediamineH2O2 was used as substrate. The reaction was stopped with sulphuric acid 12%. Optical density was recorded at 492 nm (OD492) in a microplate reader (Thermo Scientifics Multiskan FC). Structural protein characterization was carried out by ELISA using four different monoclonal antibodies (MAbs 1-5, 2-4, 3-3 and 3-2) directed to conformational epitopes of FMDV A/Arg/ 01 [12]. Lysates of transfected cells were loaded onto a 45% - 15% sucrose gradient and centrifuged at 50,000 g 16 h in a SW28 rotor at 4˚C. Fractions were collected and tested by ELISA. Inactivated FMDV A/Arg/2001 was run at the same conditions and used as positive control.
3. RESULTS AND DISCUSSION
The polyprotein P12A and protease 3C genes from FMDV serotype A/Arg/01 were amplified and cloned into the pCI-neo vector. Immunoprecipitation and Western blotting were used to determine the correct expression of the poliprotein P12A and protease 3C after transient transfection. As shown in Figure 1 the P12A precursor was successfully cleaved by protease 3C into the structural proteins VP0 (37 kDa), VP3 (23 kDa) and VP1 (23 kDa).
In order to determine if the structural proteins assembled into subviral particles, the lysates of cells transfected with pCI-P12A3C were analyzed by ELISA using MAbs directed to conformational epitopes of FMDV A/Arg/2001 [12]. The recombinant proteins and the virus were recognized by the four assayed MAbs (Figure 2(a)) suggesting that the structural proteins VP0, VP3 and VP1 assembled together into subviral particles.
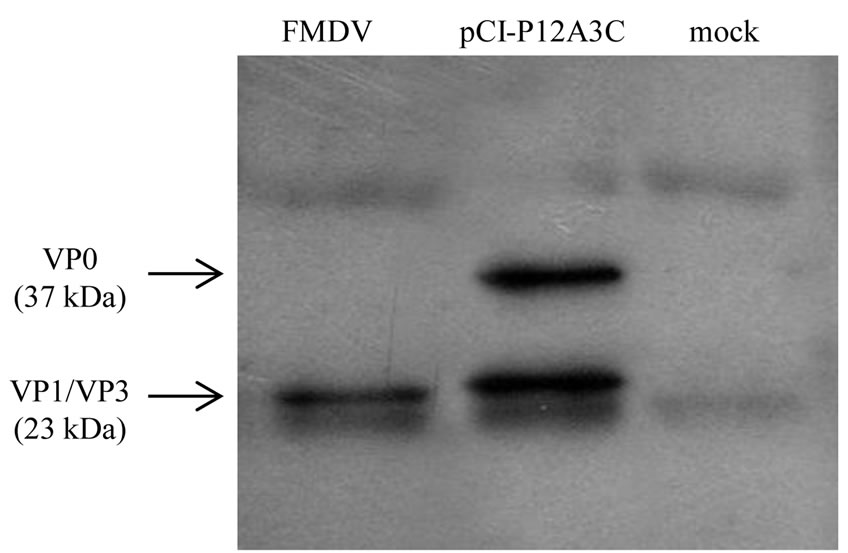
Figure 1. Western blot analysis of recombinant proteins immunoprecipitated with an anti-FMDV mouse serum. Protein extracts were run on SDS-page, transferred onto a nitrocellulose membrane, and a guinea pig serum against FMDV was used as primary antibody.
Figure 2. (a) Antigenic reactivity of purified FMDV and lysates of pCI-P12A3C or mock-transfected cells with MAbs: 1-5, 2-4, 3-2 and 3-3; (b) Inactivated FMDV and lysates of pCI-P12A3C or mock-transfected cells were loaded onto a 45% - 15% sucrose gradient. Fractions were collected and analyzed by solid phase ELISA. Known positions of the FMDV virions (146S), empty capsids (75S), pentamers (12S) and protomers (5S) are indicated.
Sedimentation in a 45% - 15% sucrose gradient was used to further analyze the ability of structural proteins to assemble into particulate subunits. Even though we were able to detect protomers (5S) and pentamers (12S) (Figure 2(b)), it was not possible to detect empty capsids in the lysates of transfected cells.
For the production of stable clones, BHK-21 cells were transfected with pCI-P12A3C and after selection and terminal dilution; the emerging clones were analyzed by ELISA for protein expression.
Three out of 36 clones were positive for recombinant protein expression (Figure 3(a)). The polyproteinP12A and protease 3C genes were confirmed to be correctly integrated into genomic DNA of these clones by PCR amplification (Figure 3(b)).
A simple co-transfection assay was used to evaluate the effect of protease 3C on protein expression in BHK-21 cells. EGFP expression decreased dramatically in cells co-transfected with pCI-3C but not in cells co-transfected with pCI-neo (empty vector) (Figure 4(a)). Also, co-transfection with pCI-3Ca resulted in a
Figure 3. Analysis of stably transfected clones. (a) Lysates of G418 resistant clones were analyzed by ELISA for the expression of recombinant proteins. The cut-off value was calculated as mean O.D. 492 nm + 3 SD (black line); (b) Genomic DNA was purified and fragments of P12A (2258 bp) and protease 3C (687 bp) were PCR-amplified from positive clones N˚18, 20 and 36 (lanes 2-4). DNA from parental cells was used as negative control (lane 1) and pCI-P12A3C plasmid as positive control (lane 5). MW: DNA molecular weight marker.
decreased EGPF expression compared to expression levels when co-transfecting with pCI-3Ci (Figure 4(b)).
Thus, the activity of the protease 3C is probably impairing the isolation of high-level expressing clones. It is worth pointing out that many recombinant viral vectors encoding the P12A3C expression cassette were successfully generated [8,13,14] while others could not be isolated [15,16].
This difference could be attributed to a different susceptibility of the host cells to protease 3C. Thus, other cell lines, such as Vero and HEK 293 (in which the development of recombinant viral vector was successful), were tested with the EGFP-co-transfection assay, but the same results were observed (data not shown). This suggests that the development of stable clones in other cell lines does not seem to be an appropriate approach to increase expression levels. The strategy of cloning the cDNA sequences of individual structural proteins (VP0, VP1 and VP3) to generate stable cell lines is complicated and tedious because a sequential selection process is needed. Moreover, it has been previously reported that the assembly of subviral particles of picornavirus from individual structural proteins is less efficient than that obtained from the P12A3C expression cassette [17].
Recombinant protein yield was about 0.014 ug/ml in stable clones and 0.15 ug/ml in transiently transfected cells (Figure 5). Therefore, for the production of recombinant FMDV capsid proteins in mammalian cells, the strategy of transient gene expression should be used instead of the generation of a stable cell line. However, further studies should be done in order to increase the expression levels and make transient transfection a suitable strategy for FMD vaccine production.
Results obtained in this study show that mammalian cells are a suitable expression system for the production of subviral particles of FMDV A/Arg/01. FMDV protomers and pentamers were produced when BHK-21 cells were transiently transfected. Since the formation of
Figure 4. Analysis of the effect of protease 3C on protein expression. Mean fluorescence intensity of cells co-transfected with pCDNA-EGFP and (a) pCI-empty or pCI-3C; (b) pCI-3Ci or pCI-3Ca. Error bars represent standard deviations derived from duplicate independent experiments.
Figure 5. Comparison of expression levels in stable clones and transiently transfected cells. Lysates were analyzed by ELISA for the expression of recombinant proteins. Error bars represent standard deviations derived from triplicates independent experiments.
higher-order structures is strongly influenced by the concentration of individual proteins [18], with this system we are probably below the critical threshold concentration of pentamers that is required before capsid formation can occur.
When we compared stable and transient expression we determined that expression levels in lysates of transiently transfected cells were higher than those achieved in lysates of stable clones.
This might be due to the effect of protease 3C on the cells. However, these expression levels are still below the ones needed for vaccine development. Thus, further studies should be done to optimize expression levels achieved by transient gene expression. The use of suspension-growing cells, serum free medium and economic transfection reagents is some of the improvements that could be explored [19]. Several authors have reported high expression levels that are compatible with the development of a vaccine (0.55 - 80 mg/ml of cell culture) using transient gene expression [9]. Moreover, in the particular case of toxic proteins, transient expression seems to be the only possible approach [20]. For all the reasons mentioned above, further studies should be done in order to make transient transfection a suitable strategy for FMD vaccine production.
4. ACKNOWLEDGEMENTS
We thank Dr. Osvaldo Zabal and his group for providing valuable help on cell culture, Diego Compaired for technical assistance and Dr. María José Gravisaco and Lic. Mariela Gammella for helping with flow cytometry assays. This work was supported by grants PAE-PICT- 2001-00044 from Agencia Nacional de Promoción Científica y Tecnológica, Argentina and PE AESA201721 from Instituto Nacional de Tecnología Agropecuaria, Argentina.