A Case of Transient Advanced Atrioventricular Block after Aortic Valve Replacement, Report of a Case ()
1. Introduction
Approximately 3% - 11.8% (mean, 7.0%) of cases require permanent pacemaker implantation (PMI) due to highdegree atrioventricular block (AVB) after aortic valve replacement (AVR), and determination of conduction disturbances such as left or right bundle branch block by preoperative electrocardiography is associated with high postoperative PMI risk [1]. We experienced a case in which the patient developed high-degree AVB after AVR was performed for severe aortic stenosis (AS) with complete right bundle branch block (CRBBB). However, her pulse returned to sinus rhythm 7 days postsurgery.
2. Case Report
A 79-year-old woman, who was examined for hypertension, and AS was diagnosed in 2006. Palpitations were noticed on occasion 2 - 3 years previously and electrocardiographic abnormalities had been indicated. Electrocardiogram findings showed sinus rhythm, a heart rate of 70 bpm, left axis deviation, and CRBBB (Figure 1). The patient treated heart failure in July 2011. Blood pressure was 106/60 mmHg, and a Levine III/IV systolic murmur was detected in the 2RSB. Laboratory dates were within normal levels. The chest radiographic imaging showed a cardiothoracic ratio of 53%. Cardiac echocardiography revealed left ventricular end-diastolic dimension/endsystolic dimension; 53/32 mm, left ventricular ejection fraction; 70%, maximum aortic valve pressure gradient/ mean gradient; 96/52 mmHg, aortic valve area; 0.89 cm2 and mild aortic regurgitation. Cardiac catheterization showed left ventricular aortic pull-back pressure; 66 mmHg, aortic regurgitation 2˚, and no significant stenosis in the coronary artery. We diagnosed severe AS and
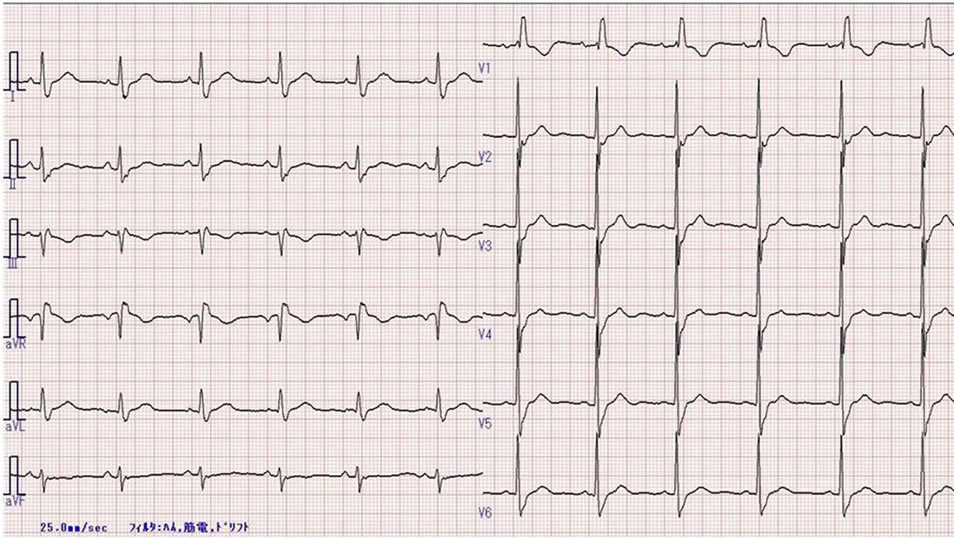
Figure 1. Preoperative electrocardiogram. Confirmed left axis deviation and complete right bundle branch block. PR interval, 0.156 s, QRS width, 0.150 s.
performed AVR. The operation was performed via the median sternotomy. After cardiac arrest, we chose a 19 mm Carpentier-Edwards PERIMOUNT MAGNA EASE TFX and we transplanted the biological valve to the intra-annular region using an everting mattress suture technique. The total surgical time was prolonged by 189 min for the cardiopulmonary bypass time and 154 min for the aortic clamp time. Because the patient was an elderly woman with calcification in the annuloaortic area and bleeding from the incision line at the ascending aorta. After the surgery peak creatine kinase (CK), peak aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) levels were 1781 IU/L, 60 IU/L, and 463 IU/L, respectively. Her own beat was not detected after the aortic clamp off, indicating high-degree AVB; therefore, the patient was weaned off the cardiopulmonary bypass using temporary ventricular pacing. On electrocardiography, we confirmed a P-wave rate of 75/min post-operatively but no following QRS wave was noted (Figure 2). She was weaned off the respirator 12 hours post-operatively. However, there was still no evidence of the QRS wave after the P wave, so we applied transvenous temporary ventricular pacing. We also considered a permanent pacemaker and managed her condition, finally her pulse returned to sinus rhythm on day 7 postoperatively, and she was then discharged from the hospital.
3. Discussion
Reports suggest that approximately 3% - 11.8% (mean, 7.0%) cases require a PMI due to high-degree AVB after AVR [1]. Damage or functional pressurization of the conduction system, which consists of the subcommissural region between the right and left coronary cusps, causes AVB. Matthews [1] analyzed 7 reports [2-6] of the relationship between AVR and permanent PMI and reported that confirmation of right or left bundle blocks and 1-degree AVB on preoperative electrocardiography are risk factors for postoperative PMI. Erdogan et al. [2] and Elahi et al. [3] reported that other risk factors in-

Figure 2. Postoperative electrocardiogram. Ventricular pacing rhythm and P wave was confirmed at 73/min, but no QRS was detected following P wave.
cluded aortic valve calcification and bicuspid valve, as well as perioperative factors, including prolonged duration on cardiopulmonary bypass time and aortic clamp time. Operative procedures can affect the conduction system during removal of calcification in highly calcified annular in AS. On the other hand, Limongelli et al. [4] and Dawkins et al. [5] reported that aortic regurgitation (AR) is a risk factor, and it remains unclear whether AS or AR is more likely to occur after postoperative AVB. Bagur et al. [7] conducted a prospective study of 780 AS cases that underwent AVR and found that the prevalence of early PMI after AVR was 3.2%, with risk factors such as left bundle block (odds ratio [OR], 4.65) and right bundle block (OR, 4.21). Although PMI increases the hospitalization duration, they reported no difference in mortality rate or 5-year survival. Totaro et al. [6] reported that a continuous suture technique is more likely to cause AVB compared to the interrupted suture technique.
If we examine the data described above, this patient was confirmed preoperatively for left axis deviation and CRBBB, as well as a high possibility of left branch bundle block, which confers a high risk of postoperative AVB. Intraoperative risk factors included calcification of the annular, prolonged duration on the cardiopulmonary bypass time and aortic clamp time may be relevant to postoperative AVB. We inferred that the pulse recovery was due to recovery of the His bundle or disappearance of the myocardial edema.
Recently, transcatheter aortic valve implantation (TAVI) has been used to manage AS; however, the analysis of Bates et al. [8] of 14 studies on post-TAVI PMI suggested that the rate of PMI after TAVI was high at 14.2%. Compared to 5.4% for the Edwards Sapien valve (Edwards Lifesciences Inc, Irvine, CA) group, the rate was 20.8% for the CoreValve (CoreValve Inc, Irvine, CA) group, indicating a significantly higher rate. Jilaihawi et al. [9] suggest that there was increased stress to the left branch of the His bundle because the stent region of the CoreValve is long and self-extending.
Although our patient’s pulse returned to sinus rhythm 7 days after the surgery, a 4 - 10 day window after AVB is most common for PMI[1], and the European Society of Cardiology recommends permanent PMI if complete AVB continues for 7 days after surgery[10].
4. Conclusion
We performed AVR for a severe AS with CRBBB and experienced the onset of temporary but severe postoperative high-degree AVB. We also reported that permanent PMI as a common complication of both AVR and TAVI.
NOTES
#Corresponding author.