1. INTRODUCTION
Gamma/delta T cells (γδТ cells) are among the first lymphoid cells appearing in ontogenesis. Many of them reside at the basolateral surfaces of intestinal epithelial cells [1]. From alpha/beta T lymphocytes, appearing a little later, γδТ cells differ by their capacity to recognize unprocessed antigens. It was shown that γδТ cells participated in the processes of oral tolerance [2] and possess immunoregulatory functions [3,4]. However, the interactions of γδТ lymphocytes with other cells are studied rather scanty, and their functional role in the intestine is not yet fully elucidated.
It was suggested that the switch from IgM to IgA in T-independent intestinal B-1 cells might be provided with γδТ cells factors. This suggestion was not experimentally checked, and the question remains open till now. The answer may be obtained by the study of the direct B-1 and γδТ cell interactions in vitro. To perform such experiments purified B-1 and γδТ cells should be used.
It is known that peritoneal B-1 cells may migrate to the gut where many of them become IgA-producers [5]. Thus, peritoneal cavity may be used as a source of B-1 cells. Method of B-1 cell isolation is well developed routine procedure. On the contrary, the isolation of γδТ cells from mouse intestine meets with significant difficulties. We tried to modify and simplify the method of mouse intestinal γδТ cell isolation.
2. MATERIALS AND METHODS
2.1. Mice
Female CBA mice, 16 - 18 g were used. Mice were maintained in the animal house of Mechnikov Institute of Vaccines and Sera of Russian Academy of Medical Science.
2.2. Isolation of γδТ Cells
Intraepithelial lymphocytes (IELs) were isolated from the small intestine; γδТ cells were isolated from IELs. To isolate IELs in some experiments collagenase of Clostridium histolyticum (Gibco) was used.
2.3. Flow Cytometry
The different cell populations were analyzed by cytometry. IELs and γδТ cells were prestained with antiCD16-32 mAb (2.4G2) to block FcγRII/RIII receptors and stained at 4˚C for 30 min with the following fluorochrome conjugated antibodies: FITC-anti-CD3 (17A2), PE-anti-CD19 (1D3), PE-anti-γ/δT (GL3) (all reagents, BD-Pharmingen).
After washing away the excess of reagents stained cells were resuspended in staining medium containing 5 μg/ml propidium iodide (PI) (Sigma). Cells stained with PI (dead cells) were excluded from analysis. Cells were analyzed on Beckman Coulter EPICS XL. Results were treated with the Programmed SYSTEM II (Beckman Coulter).
3. RESULTS AND DISCUSSION
Over the years, a number of strategies have been developed to obtain enriched preparations of intraepithelial lymphocytes (IELs) and γδТ cells. The method described are used mainly for isolation of human intestinal cells [6], although the methods of the isolation of IELs and T lymphocytes from monkey [7], rat [8], pig [9] and mice intestine are also described [10].
The most commonly used approaches involve the removal and washing of intestine, isolation of IELs and purification of T lymphocytes through Percoll density gradients. There are different modifications of these approaches concerning mainly the mode of tissue degradation, the time and the temperature of incubation of intestine segments with EDTA and dithiotreitol (DTT) and the concentrations of collagenase.
We have found that low viability of mouse IELs depended mainly on the high sensibility of intestinal mouse cells to the temperature and, especially, to the collagenase treatment. In addition, relatively many IELs (and γδТ cells, respectively) were lost during centrifugation in Percoll gradients. Therefore we tried to develop the method of IEL isolation without collagenase treatment and without cell purification through Percoll gradient. Below the protocol used in our experiments is written in details.
Mouse small intestine was washed by RPMI 1640 medium with 8% FCS on ice, everted and cut into several 2 - 3 cm segments to avoid winding. (Note! The intestine should not be minced or cut into very small pieces, otherwise the yield of cells would be greater but their viability would be less). The segments were placed into a 50-ml conical tube containing RPMI 1640 medium with 8% FCS, 1 mM EDTA, 1 mM DTT (Sigma), and 250 μl of penicillin + streptomycin (1/100) (Pen Strep, Gibco). The tube was shaken horizontally on an orbital shaker for 30 - 60 min at 37˚C. The tissue suspension was passed through 4 layers of gauze to remove mucus and centrifuged for 6 min. at 400 g. The yield of IELs CD3+ T cells and γδТ cells after different time of isolation is present in the Figure 1. It may be seen that the increase of the time of tissue incubation from 30 to 60 min. permits to isolate from the intestine of 3 mice about 38 - 40 × 106 cells instead of 1 - 2 × 106 after 30 min. incubation.
To purify γδТ cells commercial ТCR γ/δT cell Isolation Kit (Miltenyi Biotec) was used. The yield of CD3+ T cells in 2 experiments was equal to 27 and 20 mln, and the purity of CD3+ T cells was about 63% - 67%. The yield of γδТ cells was 1.0 - 1.2 × 106 (from
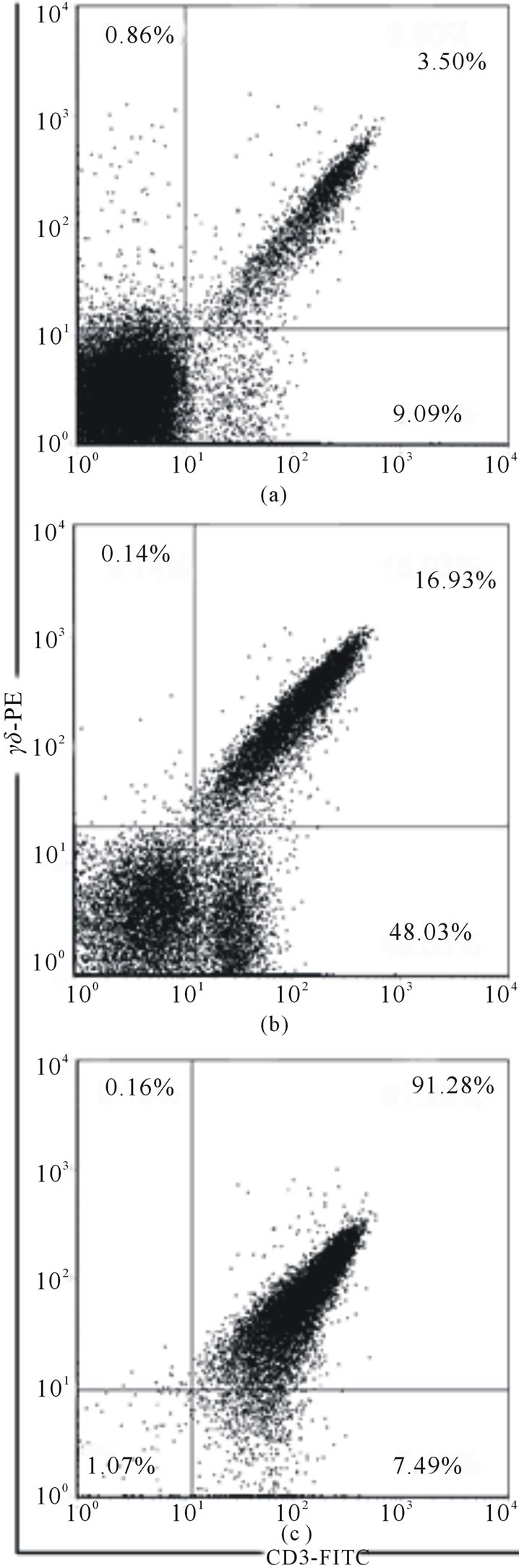
Figure 1. The percentage of γδТ cells at different stages: (a) after 30 min of incubation; (b) after 60 min of incubation; (c) after magnetic cell sorting.
intestines of 3 mice). After one-step purification the purity of γδТ cells was about 50%, but after two step purification it increased to 90% - 92%. The viability of cells was not less of 85%. These data do not differ from those recently published [11,12], where IELs were purified by centrifugation through Percoll gradient, and γδТ cells were isolated by immunomagnetic method. We believe that modification described here may be used for the isolation of γδТ cells for in vivo and in vitro studies.
4. CONCLUSION
The method of γδТ cell isolation from mouse small intestine without collagenase treatment and without centrifugation through Percoll gradients is described. The procedure permits obtaining viable γδТ cells of 90% purity.