Garlic Clove Catalyzed Biginelli Reaction in Water at Ambient Temperature ()
1. Introduction
Pyrimidines and their derivatives play an important role in human vital functions. The pyrimidine skeleton is the component of a series of natural compounds (vitamin B1, nucleic acids), chemotherapeutic drugs (Flurouracil) and synthetic medicines (Barbiturates). The biological importance of pyrimidine derivatives caused a significant interest in their synthesis [1-3]. Dihydropyrimidines (DHMPs) and their derivatives are pharmaceutically important as Calcium channel blockers, α1-1-a-antagonists, antihypertensive agents, inhibitors of the fatty acid transporter, and mitotic kinesin inhibition [4,5]. These compounds have also been found to posses antiviral, antitumor and antibacterial properties [6]. Moreover, the biological activity of some isolated marine natural products and alkaloids have been attributed to the dihydropyrimidine moiety [7]. For example, the anti-cancer agent monostrol (Figure 1) has been shown to specifically effect mitosis via a new mechanism consisting of the specific and reversible inhibition of the motiliry of the motor protein mitotic kinesin [8-10].
Since more than one hundred year, Biginelli suggested a dihydropyrimidine ring construction based on the use of β-dicabonyl compounds as a source of two carbon fragment with aldehydes and urea or thiourea as a N-C-N fragment [11]. It is worth mentioning that Biginelli reaction is one of the most named reactions and his collaboration is still considered one of the important pyrimidine synthesis. In the past 10 years, several one-pot methodologies for the synthesis of DHPM derivatives were developed and several modifications have been introduced. Most of them are based on Lewis acid-catalyzed reactions [12-19] which permits the reaction to proceed under milder conditions and with higher yields, than those outlined by Biginelli in the original procedure. Microwave irradiation has also proved beneficial [20]. Natural acidic catalysts have been also utilized [21]. Very recently, Biginelli reaction has been conducted under basic conditions. This involves the use of PPh3, under solvent free conditions [22], t-BuOK at 70˚C [23], chiral primary amines [24] and ammonium carbonate in water [25].
It is worth mentioning that many of these existing methods displayed drawbacks, such as environmental pollution caused by utilizing catalysts in stoichiometric
quantities, exotic reaction conditions, unsatisfactory yields and complicated operations while others posses some advantages overcoming these drawbacks. With the aim for the development of environmentally friendly technique, we investigated herein the utility of bio-catalysis in Biginelli reaction at ambient temperature. To the best of our knowledge, such approach has not been reported in the literature. Employing bio-catalysis is advantageous since it is green, proceeded at ambient temperature and is attractive from economical, environmental and handling point of view. In continuation of our work on the synthesis of azines and fused azines [26-28], we report herein the first attempt to synthesis DHMPs 4 using aryl aldehydes 1, ethyl acetoacetate 2, urea or thiourea 3 catalyzed by crushed garlic clove at ambient temperature (Scheme 1).
2. Results and Discussion
The structure of the products 4 were confirmed by comparison (TLC) with authentic samples [29-33], prepared by refluxing the reaction mixture in ethanol containing 1 mL of hydrochloric acid. In order to examine the effect of substituted aryl aldehyde 1 on the reaction rate and overall yield, various functionalized aryl aldehydes were used under the above reaction conditions. It has been found that the reaction proceeds smoothly to give DHMPs 4 in high yields with a slight decrease in the yield when the aryl substituent involves a strong electron donating group (cf. Table 1).
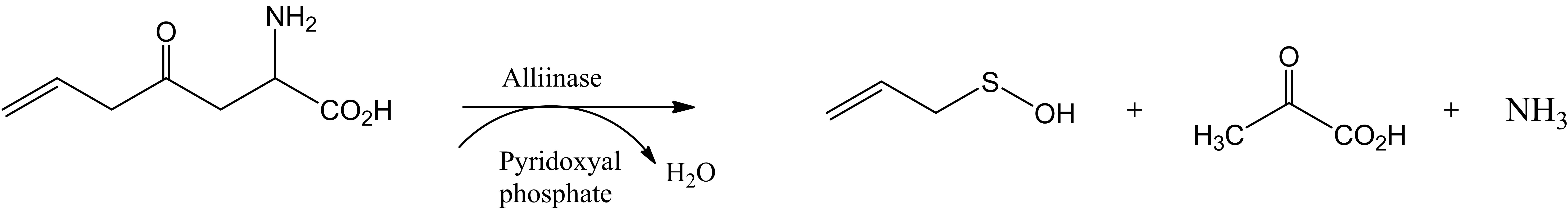
A proposed mechanism to account for the formation of 4 is demonstrated in Scheme 2 which involves enzymatic transformation of allin[(+)-(S)-allyl-cysteine-sulfoxide] influenced by alliinase producing allyl sulfenic acid, pyruvic acid and ammonia. Both could catalyze Biginelli reaction in the well established mechanism either acidic or basic conditions. At room temperature, these enzymatic transformations occur in 10 - 15 minutes (Scheme 2).
3. Conclusion
We have successfully developed an easy, high yielding and versatile protocol for the synthesis of DHMPs from the reaction of aryl aldehydes, ethyl acetoacetate, urea or thiourea catalyzed by crushed garlic clove at ambient temperature. The process does not require the use of any hazardous or harmful catalysts and thus it is a simple, environmentally friendly technique of high atom economy and yields.

Scheme 1. Synthesis of dihydropyrimidines.
Scheme 2. A proposed mechanism for the formation of dihydropyrimidines 4.
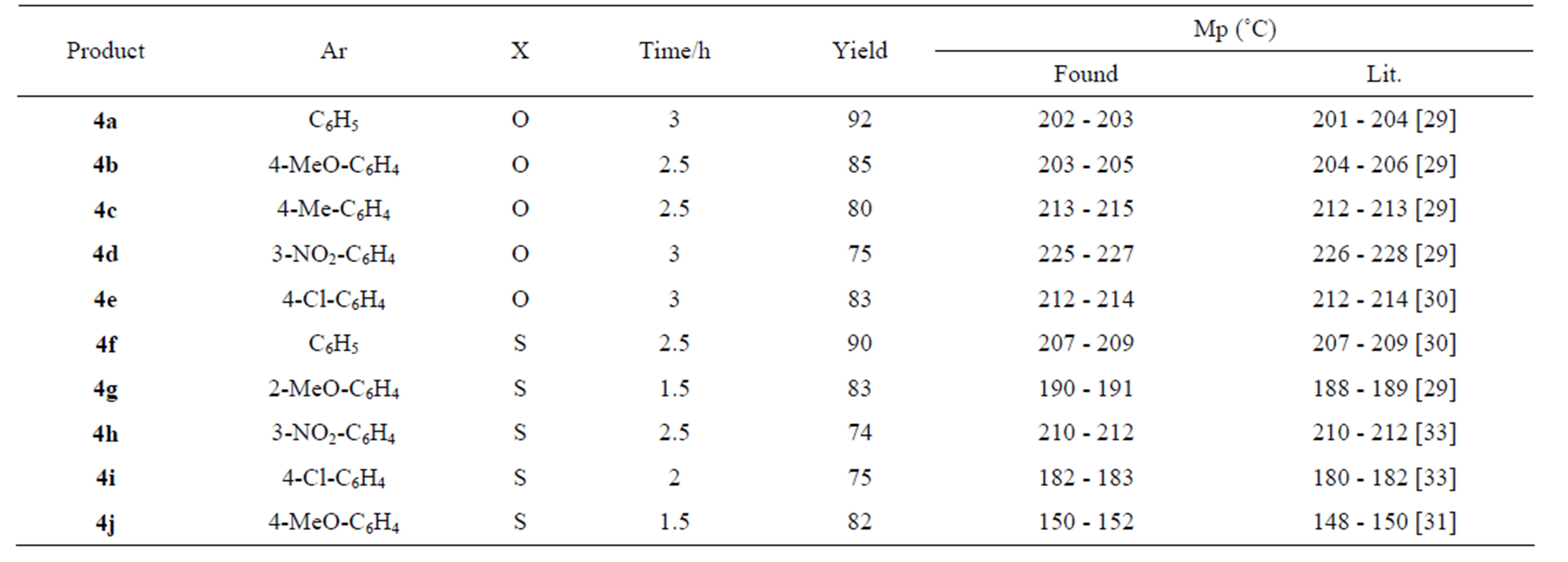
Table 1. Garlic clove synthesis of Biginelli DHMPs 4a-j.
4. Experimental
4.1. General
Melting points were measured on a Gallenkamp apparatus and were not corrected. IR spectra were recorded with a Schimadzu 470 spectrophotometer in KBr disks. Peaks are reported in cm−1. 1H and 13C NMR spectra were recorded on a Bruker AM400 (400 MHz for 1H NMR and 100 MHz for 13C NMR) spectrometer in DMSOd6 using TMS as an internal standard; the chemical shifts are given in δ units (ppm). Mass spectra were measured on a GCMS-QP1000EX (EI, 70 eV) mass spectrometer. Analytical thin-layer chromatography (TLC) was performed on aluminum sheets precoated with silica gel (Merck, Kieselgel 60F-254). Visualization was accomplished by UV light. Micro-analyses were performed at the Microanalytical Data Unit at Cairo University and analytical values obtained were within ±0.4% of the calculated values.
4.2. Synthesis of Dihydropyrimidines 4a-j; General Procedure
To a mixture of each of aldehydes 1a-j (10 mmol), ethyl acetoacetate 2 (10 mmol) was added crushed garlic glove (100 mg every 15 min.). Then, the reaction mixture was stirred at room temperature (25˚C) for the cited time (TLC) control. Then, the reaction mixture was dissolved in EtOH, filtered and the filtrate was concentrated under reduced pressure. The resulting solid product was collected by filtration, dried and recrystallized from EtOH.
4.3. Ethyl 6-methyl-2-oxo-4-phenyl-1,2,3,4- tetrahydro-5-pyrimidine carboxylate (4a)
1H NMR (400 MHz, DMSO-d6): d = 1.12 (t, J = 7.2 Hz, 3H), 2.33 (s, 3H), 4.02 (q, J = 7.2 Hz, 2H), 5.08 (d, J = 4 Hz, 1H), 6.35 (t, J = 7.5 Hz, 2H), 6.87 (t, J = 7.3 Hz, 1H), 7.33 (d, J = 7.5 Hz, 2H), 9.66 (s, 1H), 10.44 (s, 1H). 13C NMR (100 MHz, DMSO-d6): d = 13.2, 61.5, 111.2, 127.8, 128.2, 130.7, 158.5, 166.0. IR (KBr): ν = 3231, 3207, 1689, 1664 cm−1. MS: m/z = 278 (M+, 100). Found: C, 64.63; H, 6.22; N, 10.87; anal. calcd for C14H16N2O3: C, 64.60; H, 6.20; N, 10.76.
4.4. Ethyl 4-(4-methoxyphenyl)-6-methyl-2-oxo- 1,2,3,4-tetrahydro-5-pyrimidine carboxylate (4b)
1H NMR (400 MHz, DMSO-d6): d = 1.12 (t, J = 7.2 Hz, 3H), 2.22 (s, 3H), 3.73 (s, 3H), 4.20 (q, J = 7.2 Hz, 2H), 5.09 (d, J = 3.2 Hz, 1H), 6.88 (d, J = 8.4 Hz, 2H), 7.14 (d, J = 8.4 Hz, 2H), 9.60 (s, 1H), 10.15 (s, 1H). IR (KBr): ν = 3230, 3204, 1688, 1665 cm−1. MS: m/z = 290 (M+, 100). Found: C, 61.96; H, 6.27; N, 9.59; anal. calcd for C15H18N2O4: C, 62.05; H, 6.24; N, 9.64.
4.5. Ethyl 4-(4-methoxyphenyl)-6-methyl-2- thioxo-1,2,3,4-tetrahydro-5-pyrimidine carboxylate (4j)
1H NMR (400 MHz, DMSO-d6): d = 1.12 (t, J = 7.1 Hz, 3H), 2.29 (s, 3H), 3.72 (s, 3H), 4.00 (q, J = 7.1 Hz, 2H), 5.06 (d, J = 3.5 Hz, 1H), 6.92 (d, J = 8.5 Hz, 2H),7.13 (d, J = 8.5 Hz, 2H), 9.22 (s, 1H), 10.32 (s, 1H). IR (KBr): ν = 3230, 3205, 1688, 1664 cm−1. MS: m/z = 306 (M+, 100). Found: C, 58.76; H, 5.82; N, 9.23, S, 10.52; anal. calcd for C15H18N2O3S: C, 58.80; H, 5.92; N, 9.14, S, 10.47.
NOTES