Molecular cloning and characterization of an ECTO-NOX3 (ENOX3) of Saccharomyces cerevisiae ()
1. INTRODUCTION
In response to a stress, plants can synthesize and secrete a large variety of secondary metabolites. We have previously shown that a wound healing and desiccation of plant tubers promoted secretion of cytokinin-like products. At least one of these compounds, kinetin riboside, induced a cytotoxic effect both on plant and mammalian tumour cells [1]. In Jerusalem artichoke tubers (Helianthus tuberosus), after the same stimuli, we have shown secretion of a cytotoxic protein related to a superoxide dismutase [2]. This protein alone or possibly associated with a co-factor was shown to induce cytotoxicity in plant and animal tumour cells, preserving non-tumour cells in both cases. In potato (Solanum tuberosum), after such a stress condition, tuber parenchyma was shown to secrete new types of Kunitz-like protease inhibitors closely associated with another unknown polypeptide of approximately 40 kDa [3].
Patatins are potato tuber proteins with acyl-hydrolyzing activity (LAH), and patatin catalytic domain is widely spread in bacterial, yeast, plant and animal enzymes. Recent results have indicated that patatin-related enzymes are involved in different cellular functions, including plant responses to auxin, elicitors or pathogens, and abiotic stresses and lipid mobilization during seed germination [4]. Individual types of patatin varying in their masses occur in patatin family in a ratio specific for each of the cultivars, with the lowest mass type being the major one. Patatin samples within most of cultivars exhibited high values of specific LAH activity. It may be supposed that individual patatin forms do not have similar physiological roles. These findings have supported the concept that patatin is not only a storage protein but could also be a part of potato defense mechanism [5].
Patatin glycoprotein is found associated with Kunitz-like protease inhibitors in mature potato tubers [6,7]. Patatin is a 42-kDa glycoprotein with various isoforms, which has been found in all Solanum species, with a precursor form containing a 23 amino-acid signal peptide [6,8-10]. In Solanum tuberosum, patatin is a multigene family with 10 to 18 members per haploid genome [11,12]. Patatin is now presumed to play an active role in plant signalling involving its lipid acyl hydrolase activity, related to the phospholipase A2 [13,14]. Indeed, it exhibits esterase activity in a broad range of lipid substrates [15]. Phospholipases A2s are esterases that cleave glycerophospholipids at the sn-2 position, releasing a fatty acid, such as arachidonic acid, and a lysophospholipid that has been found to perform many important roles both in plants and animals [16-19]. A patatin-like phospholipase family was recently described in humans, which indicates that patatin or patatin-like peptides may have conserved an activity in cell signalling across both the animal and plant kingdoms [20]. Phospholipid degradation initiated by receptor stimulation produces various lipid mediators that relay information from extracellular signals to intracellular events [19]. In animals, PLA2 enzymes and their enzymatic products have been implicated in diverse cellular responses, including bioactive lipid production, inflammation, and signal transduction [17,18]. PLA2 activities have also been implicated in cellular process in plants, such as growth, development, stress responses, and defence signalling [21].
Patatin domain-containing lipolytic enzymes, according to several authors, now comprise five subfamilies: plant patatin-related enzymes, animal calcium-independent enzymes (iPLA2α, iPLA2β, iPLA2γ), calciumactivated (cytosolic) cPLA2 enzymes, neuropathy target esterases (NTEs) and desnutrin-related triglyceride hydrolases [4]. pPLA-IIα is not only a signaling enzyme but can catalyze a late, non-specific hydrolysis of membrane lipids that commits tissues to cell death and affects differential resistance to pathogens. Members of the patatin LAH family can have opposite effects on pathogen resistance, possibly via distinct molecular mechanisms [22]. Growing evidence suggests that phospholipase A2 plays a pivotal role in tumorigenesis in human gastrointestinal cancer. One of the well-studied isoforms of PLA2, group IIA PLA2 (PLA2G2A) appears to exert its protumorigenic or antitumorigenic effects on a tissuespecific manner. PLA2G2A protein is an extracellular enzyme with a low molecular mass of ~14 kd. Presence of PLA2G2A has been observed in a variety of human diseases including cancer [23].
In this work, we show that a patatin-like PLA2 was actively secreted from potato tuber parenchyma after cutting and water stress, and that protein is involved in cytotoxicity mainly against tumour cells in plants and animals.
2. MATERIALS AND METHODS
2.1. Plant Materials
Potato tubers (Solanum tuberosum L. cv agata) of commercial origin were used as source. Jerusalem artichoke tubers (Helianthus tuberosus L.) were grown in fields, harvested in autumn and stored at 4˚C (3 months) to relieve their dormancy. The crown-gall, a well-known model of plant tumours, was induced by Agrobacterium tumefaciens (strain B6 806) infection on pieces of Jerusalem artichoke tubers (H. tuberosus L) cultured in Knop medium under controlled conditions (25˚C) as previously described [24]. This plant was chosen for its high susceptibility to infection by A. tumefaciens. Tumour tissue can be obtained 10 to 15 days following inoculation. Cefotaxime (0.5 g∙l−1) was added to the Knop medium then to eliminate agrobacteria. The tumour tissue grew on parenchyma, which was maintained outside the Knop mixture, and only non-transformed part of parenchyma was thus immersed in the medium.
2.2. Isolation and Purification of the Cytotoxic Agent
Parenchyma of potato tubers (S. tuberosum L. cv agata) was cut in parallelepipeds (length 2 cm, section 1 × 1 cm) that were left for 24 h in a sterile culture tube at 25˚C in the dark for air-drying. Parenchyma pieces were transferred into tubes containing 3 ml of sterile Knop medium, diluted two-fold, at pH 5.5 for 2 h. Only 1/3 of tuber piece was thus immersed. Then, culture medium was collected and centrifuged at 10,000 g for 15 minutes. Supernatant was collected and dialysed for 4 h against distilled water under stirring at 4˚C (Spectra dialysis membrane, porosity MWCO: 6 - 8000). Finally, mercaptoethanol (8 mM) was added to prevent protein adsorption and the solution was filtered throughout MillexTM filter device, with PVDF DuraporeTM membrane (0.22 µm; Millipore, Bedford, USA). Such a sterile solution was called fraction S1, and could be stored at 4˚C before use.
S1 fraction was then concentrated and small molecules were removed by using a centrifugal filter unit with a 10-kDa cut-off membrane (AMICON Ultra-15 10.000 MWCO, Millipore). Aliquots of 15 ml were centrifuged for 10 min at 2800 g. Fractions (2 ml) then could be sterilized by filtration as described above. Protein fractions, approximately eight-fold concentrated, were called S2. Protein content was assayed according to Bradford [25] or using NanoOrange protein assay kit according to the supplier’s protocol (Molecular Probes, Invitrogen, CergyPontoise, France).
2.3. Protein Purification
Proteins of S2 fractions were separated following their molecular weights on sephacrylamide (S300-HR, Sigma) column as previously described [3]. Polypeptides were eluted with phosphate buffer solution (0.2 M, pH 7.5) and fractions of 3 ml or 1 ml were then recovered. Since patatin is a mannosyl glycoprotein, it can be precipitated by specific lectins, such as concanavalin A (0.5 mg·ml−1). Following centrifugation (15 min, 12,000 g), pellet was dissolved again and run through concanavalin A column. Patatin-like polypeptides were finally removed from the support by 4% Methylα-d-mannopyranoside solution.
2.4. Polyacrylamide Gel Electrophoresis (PAGEs)
Non-denaturing PAGE was performed according to Clarke and Critchley [26]. Protein samples were separated on 10% polyacrylamide gel in Tris-glycine buffer (pH 8.3), with 4% acrylamide for stacking gel in Trisglycine buffer (pH 6.8). After electrophoretic separation, proteins or polypeptides were removed from gels by electro-elution (Biotrap System, Schleicher and Schuell), for 4 h under 200 V in a Tris-glycine buffer (Tris-HCl 25 mM and glycine 192 mM). Samples were sterilized through Millipore filters (0.22 µm). Protein mixtures thus could be stored at 4˚C.
2.5. 1Dor 2D-SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGEs)
Polypeptides were separated by SDS PAGE (in Apelex model V10-CDC apparatus), according to Laemmli [27] modified by Schagger and Von Jagow [28]. Protein samples were mixed with an equal volume of dissolving solution (5% v/v 2-mercaptoethanol, 10% w/v glycerol, 4% w/v SDS and 0.01% w/v bromophenol blue, in 0.5 M Tris-HCl buffer pH 6.8), then heated in boiling water for 2 min. An equal volume (5 µl) for each sample was loaded on 12.5% or 15% polyacrylamide gels (4% acrylamide for the stacking gel in a SDS-Tris-glycine buffer pH 6.8) in SDS-Tris-glycine buffer pH 8.8. Gels were stained either with silver nitrate according to Blum et al. (1987) [29], or with Coomassie Blue R250 (BioRad) [30].
Two-dimension isoelectrofocusing electrophoresis (2-D IEF PAGE) was performed according to O’Farrell [31]. Non-equilibrium pH gradient electrophoresis was done with fraction of secreted patatin-like proteins previously purified by PAGE in non-denaturing conditions [3]. First dimension was done in polyacrylamide gel (6.4% acrylamide) containing ampholines (pH 3.5 - 10 (250 µl) or 5 - 7 (450 µl): Pharmacia Biotech), at 4˚C, for 16 h (900 V); electrophoresis was realized using 0.02 M NaOH solution in cathode tank and 0.01 M H3PO4 solution at anode.
Second dimension was performed by SDS-PAGE either with 12.5% acrylamide, for separation gel, in SDSTris-glycine buffer pH 8.8 with a stacking gel (5% acrylamide in SDS-Tris-glycine buffer pH 6.8), or in 6% - 15% acrylamide gradient gel (in SDS-Tris-glycine buffer pH 8.8), for 5 h at 4˚C with 25 mA constant current (EC-Apparatus Corporation gel column 7698 and Hoefer SE600 apparatus). Gels were stained with Coomassie Blue R250. Stained spots were cut from the gel and transferred onto a PVDF membrane for polypeptide sequencing. Molecular weights were evaluated by migration of Sigma MarkerTM Low Range.
2.6. Polypeptide Sequencing
Polypeptide sequences were analysed by MALDI-TOF mass spectrometry (“Matrix Assisted Laser Desorption Ionisation-Times of Flight”). Polypeptides, purified by 2D IEF PAGE, were removed from gels and hydrolyzed with trypsin. Produced peptides were analysed in mass spectrometer (Voyager DEPRO Biosystems) and patterns were compared to Swiss Prot data bank from NCBI.
Amino-terminal polypeptide sequences were analysed after polypeptide separation by 2D-electrophoresis. Proteins were electro-blotted onto polyvinylidiene difluoride (PVDF) membrane (Bio-Rad, Marnes la Coquette, France), using semi-dry blotter system IMM-2 (The W.E.P. Company, Seattle, Wa., USA). Stained protein spots were cut off PVDF membrane, washed in distilled water and airdried before sequencing (Proseq). Amino-terminal sequences were obtained according to Edman and Begg [32], and compared with known proteins into amino acid sequence data base (NCBI, protein Blast Data base).
2.7. In Vitro Test for Antitumour Effect on Plants
Cytotoxic activities of S1, S2 or chromatography fraction solutions were tested on crown-gall tumour fragments, by direct application on tumour tissue or by dipping plant fragments into sterile Knop medium containing secreted products (dilution 1/2). Three replicates of eight tumour samples were used for each experiment.
2.8. Animal Cell Culture and Colony-Forming Assay
Anti-clonogenic effects were assayed on two murine cell lines: B16 melanoma cells and L929 immortalized fibroblastoid cells. B16 cells were obtained from the American Type Culture Collection (ATCC, Manassas, USA) and L929 from the European Collection of Cell Cultures (ECACC, Salisbury, United Kingdom). Cells were cultured in Minimum Eagle’s Essential Medium (MEM, Gibco, Invitrogen, Cergy-Pontoise, France) supplemented with 1% (v/v) vitamin cocktail, 1% glutamine, 1% non-essential amino-acids, 4 µg/ml gentamicin, and 10% foetal calf serum (Sigma, Les Abresle, France) at 37˚C in hydrated air with 5% CO2. Doubling time was around 15 h for both cell lines. B16 or L929 cells were plated into Petri dishes (40 mm in diameter; 150 cells/ plate) and incubated overnight before treatment. The medium was removed and fresh MEM containing either plant secretion, or purified fractions in different amounts, or control solution (NaCl 0.9% m/v) was added. Ten days later, plates were rinsed with PBS (Phosphate Buffer Saline; Invitrogen). Cells were fixed in methanol, and stained with 0.2% w/v crystal violet solution. Colonies containing more than 50 cells were counted and cell clonogenicity (percentage of cells forming colonies relative to control) was calculated. Each treatment was performed in triplicate and each experiment was at least duplicated.
2.9. PLA2 Activity Assays
PLA2 activity was evaluated by method of Radvanyi et al. [33]. Substrate used was a pyrene-associated phospholipid 1-Hexadecanoyl-2-(1-pyrenedecanoyl)sn-glycero-3- phosphoglycerol (β-pyC-10-PG; Molecular ProbesTM, Invitrogen). After phospholipid hydrolysis by PLA2 activity, the pyrene-probe free fatty acids bind tightly to BSA and fluoresces at 377 nm (excitation wavelength 342 nm) which was quantified with a Fluoroskan Ascent FL reader. Measures were kinetically done, every 20 s, for 3 min and reaction velocity was calculated by linear regression analysis. Phospholipase A2 from Naja mozambica (Sigma) was used as positive control.
PLA2 activity has been characterized using specific inhibitors like ATK (Arachidonyl trifluoromethyl ketone), PTK (Palmitoyl trifluorylmethyl ketone), BEL (Bromoenol Lactone, (E)-6-(bromomethylene)-3-(1-naphtalenyl) 2H-tetrahydropyran-2-one) and MAFP (Methyl Arachidonyl Fluorophosphonate) (Calbiochem, San Diego, USA).
3. RESULTS
3.1. Secreted Protein Characterisation
To characterize the active antitumour agent, the protein fraction of the plant secretion product was analysed. Non-denaturing PAGE of secretion products revealed three silver nitrate stained bands, two minor ones around 180 kDa (band A) and 40 kDa (band C) and a major band at 95 kDa (band B) [3]. Using SDS-PAGE, we previously described two sets of polypeptides which comigrate in band B: a major polypeptide (MW 35 - 40 kDa) and a smaller one (around 16 kDa) containing Kunitz-type protease inhibitors [3].
Analysis of the main protein spot, obtained in nondenaturing electrophoresis gel, by 2D isoelectrofocusing PAGE had displayed several polypeptide spots with close MW around 36 kDa (Figure 1 spot B1). Other

Figure 1. 2D IEF electrophoresis of patatin-secreted product Non equilibrium pH gradient elecrophoresis was realised on a fraction of secreted PLA2-like patatin previously purified and separated in polyacrylamide gel, after an electrophoresis in non-denaturing conditions. First dimension was done in polyacrylamide gel (6.4%) with ampholines (250 µl ampholines 3.5 - 10 and 450 µl ampholines 5 - 7), at 4˚C, for 16 h, at 900V; 2nd dimension was realised by SDS-PAGE (12.5% acrylamide, 4˚C, 5 h, 25 mA). Gel was stained with Coomassie Blue R250. Stained spots B 1 - 5 were cut off gel and transferred onto a PVDF sheet for sequence analysis of polypeptides. Polypeptides are described in Table 1. Molecular weights were obtained by migration of Sigma MarkerTM Low Range (M).
smaller spots, around 24 - 30 kDa (spots B2-4), were possibly stained (Figure 1). N-terminal sequence analysis of four of the main polypeptide spots, separated on 2D gels, have shown a sequence related to patatin polypeptide, but with amino-acid differences for one out of patatin spots (Table 1). Variations of sequences mainly occurred at the level of first and third N-terminal amino acid. A protease inhibitor (spot B5) often is associated to patatin complex (Figure 1) [3]. 2D IEF spots were removed from gel and analysed by MALDI-TOF method (Table 1). Spots B1, B2 and B4 were shown to contain the ion 1437 and the spot B3 to have ion 1464. Data thus demonstrate that at least two isoforms of patatin were released in our experimental conditions. Amino terminal sequences for polypeptide spots of secreted patatin displayed a nucleotide-binding consensus motif GGGIKG that was observed in iPLA2 gene family [24]. Moreover, analysed spots displayed sequence GGGIKGII described in iPLA2α sequence (40 kDa, 386 amino-acids) from mammals [34].
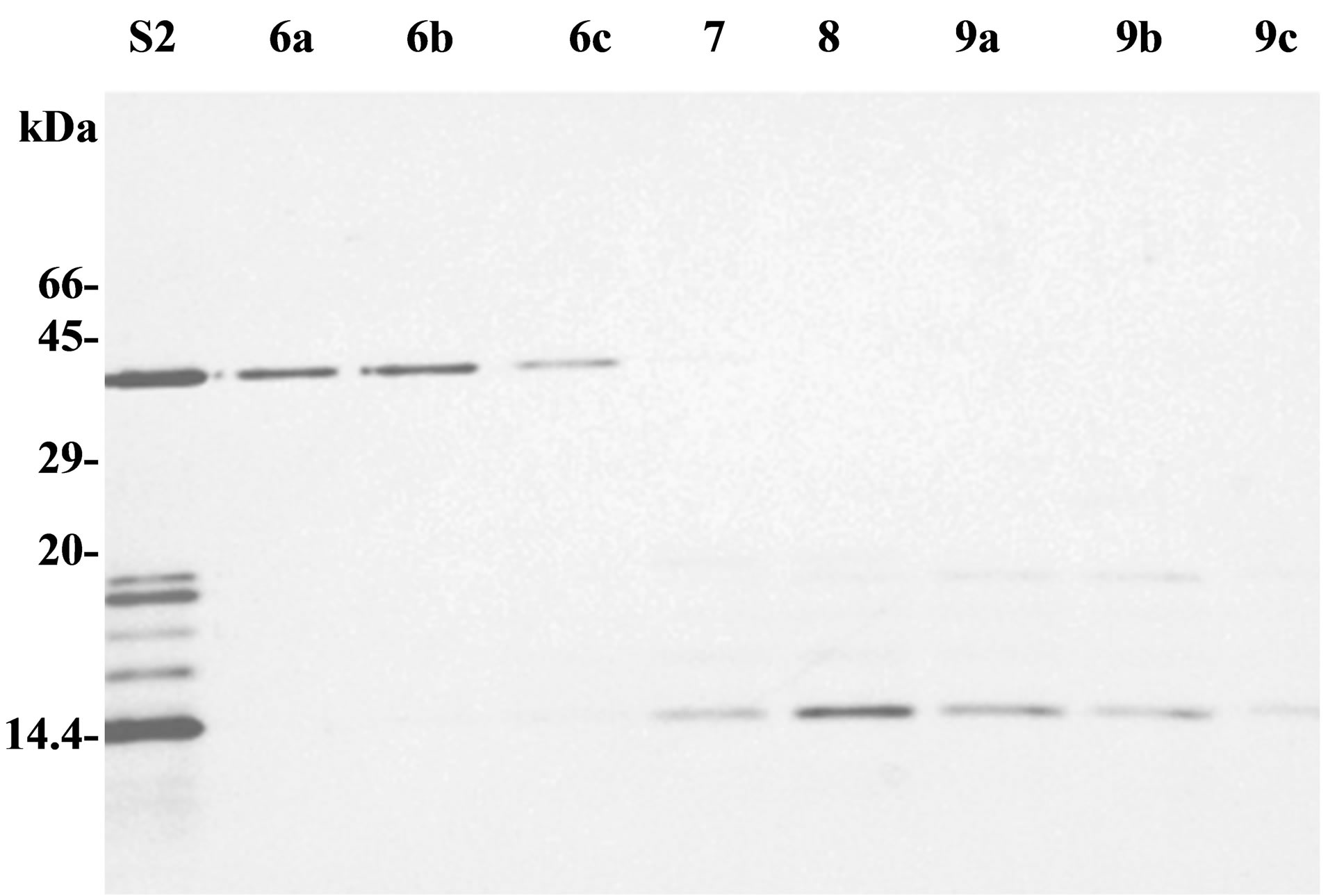
Polypeptides of secretion fraction S2 can be eventually separated by exclusion chromatography through a sephacrylamide column. Protein content of each eluted fraction was analysed by SDS-PAGE (Figure 2(a)). A 40 kDa polypeptide was found purified in fractions 6a-c (F6). Plant antitumour activity, previously observed with purified secretion products (S1 or S2), was present in fraction F6 but not in other fractions. 2D IEF-PAGE analysis of fraction F6 showed several spots between 36 and 40 kDa, with a pI ranging from 6 to 7, but did not contain any protease inhibitor spots (data not shown).
To assess whether the proteins leave the tissue passively or by secretory pathway, we compared protein patterns obtained with or without orthovanadate treatment,
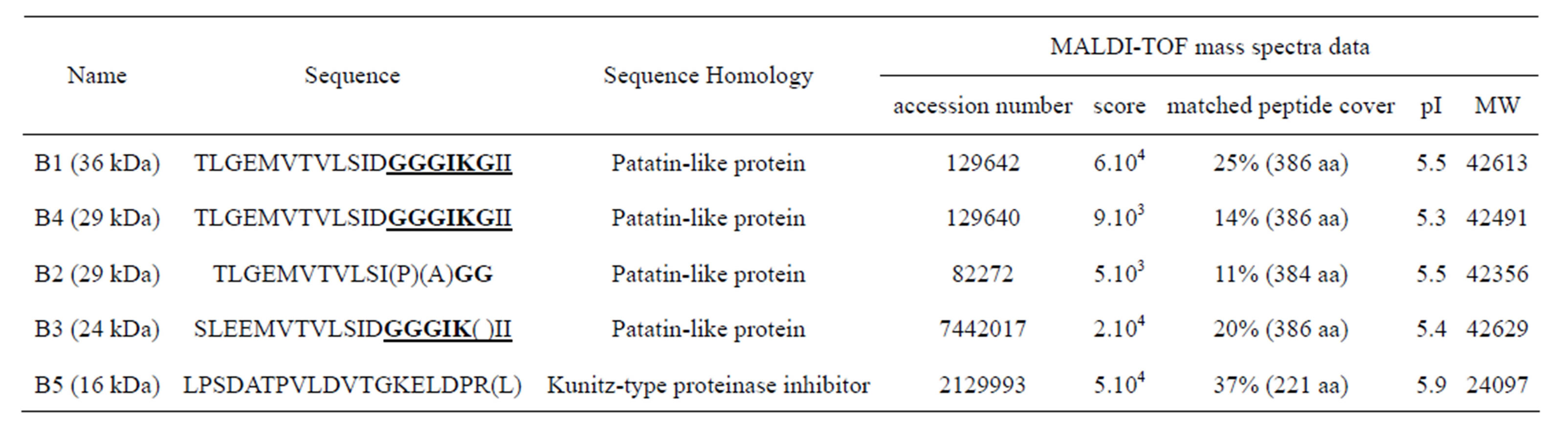
Table 1. N-terminal sequences of main polypeptides associated to the excreta of potato tuber parenchyma. Spots are called and molecular weights are observed on the 2D IEF gel in Figure 1. N-terminal sequence analysed and nucleotide-binding consensus motif GGGIKG, which is observed in the iPLA2 gene family, are displayed in bold and underlined. Molecular weights (MW) were given by database.
 (a)
(a) (b)
(b)
Figure 2. Analysis of purified secreted patatin. (a) Purified secretion product (S2), and fractions obtained from sephacrylamide column, were analysed by SDS-PAGE (15% acrylamide, 110 V, 20 h). Samples had either a volume of 3 ml (fractions 7, 8) or 1 ml (fractions 6a, 6b, 6c and 9a, 9b, 9c). (b) SDS-PAGE analysis of effect of vanadate on patatin secretion. Pieces of tuber parenchyma were put in Knop solution for 2 hours just after cutting (lanes 1 and 2) or after a 48 h drying period at 25˚C (lanes 3 and 4). Each sample was either supplemented with 1 mM orthovanadate (lanes 2 and 4) or did not contain any vanadate (lanes 1 and 3). Arrow indicates patatin polypeptides (40 kDa). SDS-PAGE was performed in 12.5% polycrylamide gel (separation gel: 12.5% acrylamide; stacking gel: 5% acrylamide; 4.5 h, 30 mA).
an inhibitor of multiple ATPases. Sodium orthovanadate is a well-known inhibitor of numerous ATPases or phosphatases, by acting as phosphate analogue [35]. In plants, orthovanadate is commonly used to inhibit ATPbinding cassette transporters [36]. When freshly cut pieces of tuber parenchyma were dipped into hydration solution with or without 1 mM sodium orthovanadate, a same polypeptide pattern was obtained in 40 kDa spot region after SDS-PAGE. Conversely, after a drying period (48 h), that allows healing up parenchyma wounds, secretion product of water-stressed tuber parenchyma displayed a different pattern after orthovanadate treatment: 40 kDa band was missing in presence of orthovanadate (Figure 2(b)). Therefore, after a drying period, polypeptide releases were not due to leakage of intracellular proteins, suggesting that patatin-like proteins might be secreted via an active, orthovanadate-sensitive process.
3.2. PLA2 Activity of 40 kDa Protein Group
To assess whether 40 kDa protein group exhibited PLA2 activity, lipid esterase activity was measured with β- pyC-10-PG, a pyrene-labeled acidic phospholipid. Since, only sn-2 phospholipid acyl chain is labelled, β- pyC-10-PG can discriminate between phospholipase A2 and phospholipase A1 activities. Pyrene fluorescence is quenched by interactions with lipid matrix and increases upon cleavage of probe by phospholipase activity. Secreted proteins (S2) induced concentration-dependent release of pyrene and this activity was seven to eight-fold higher in fraction F6 (Figure 3(a)). PLA2 activity was inhibited by ATK, PTK, and to a lesser extent by MAFP (Figure 3(b)), which are well-known inhibitors of cPLA2 and iPLA2 activities [16,35-38]. BEL up to 20 µM, an iPLA2 inhibitor [39,40], had, at most, a slight effect on PLA2 activity of secreted patatin-like protein (data not shown). Finally, addition of EGTA (100 µM) did not change PLA2 activity of secreted patatin-like protein (data not shown). This pattern of inhibitor sensitivity fits well with calcium-independent cPLA2 activity of the secreted patatin-like protein.
3.3. Secreted Product from Injured and Water-Stressed Potato Tubers Induces Anti-Crown-Gall Effect
To investigate antitumor effect of secreted product on plants, experiments were performed using crown-gall tissue obtained from H. tuberosus tubers previously infected by Agrobacterium tumefaciens. Purified protein Fraction 6 secreted by potato tubers was added locally to plant tumour fragments cut off from parenchyma or in situ. Necrosis of tumour tissue was observed two days after treatment (Figure 4). Secreted product first induced a brown colour of plant tumour tissue, and a complete necrosis was achieved after 48 h. When secreted product was applied in situ, no effect was observed on healthy parenchyma tissue. Finally, cytotoxic effect was lost when secreted proteins were denatured by precipitation with ammonium sulphate (data not shown).
Since patatin-like protein of secreted product showed PLA2 activity, as previously reported for the vacuole-stored patatin, and the latter is known to be retained on a concanavalin A column, we investigated whether this was also a feature of the secreted product. PLA2 activity was observed after purification of 40 kDa polypeptide by concanavalin-affinity chromatography (Figure 5(a), lane C). In contrast, the fraction of secretion product, that was not retained on column and deprived of 40-kDa-protein, had no significant PLA2 activity (Figure 5(a), lane N).
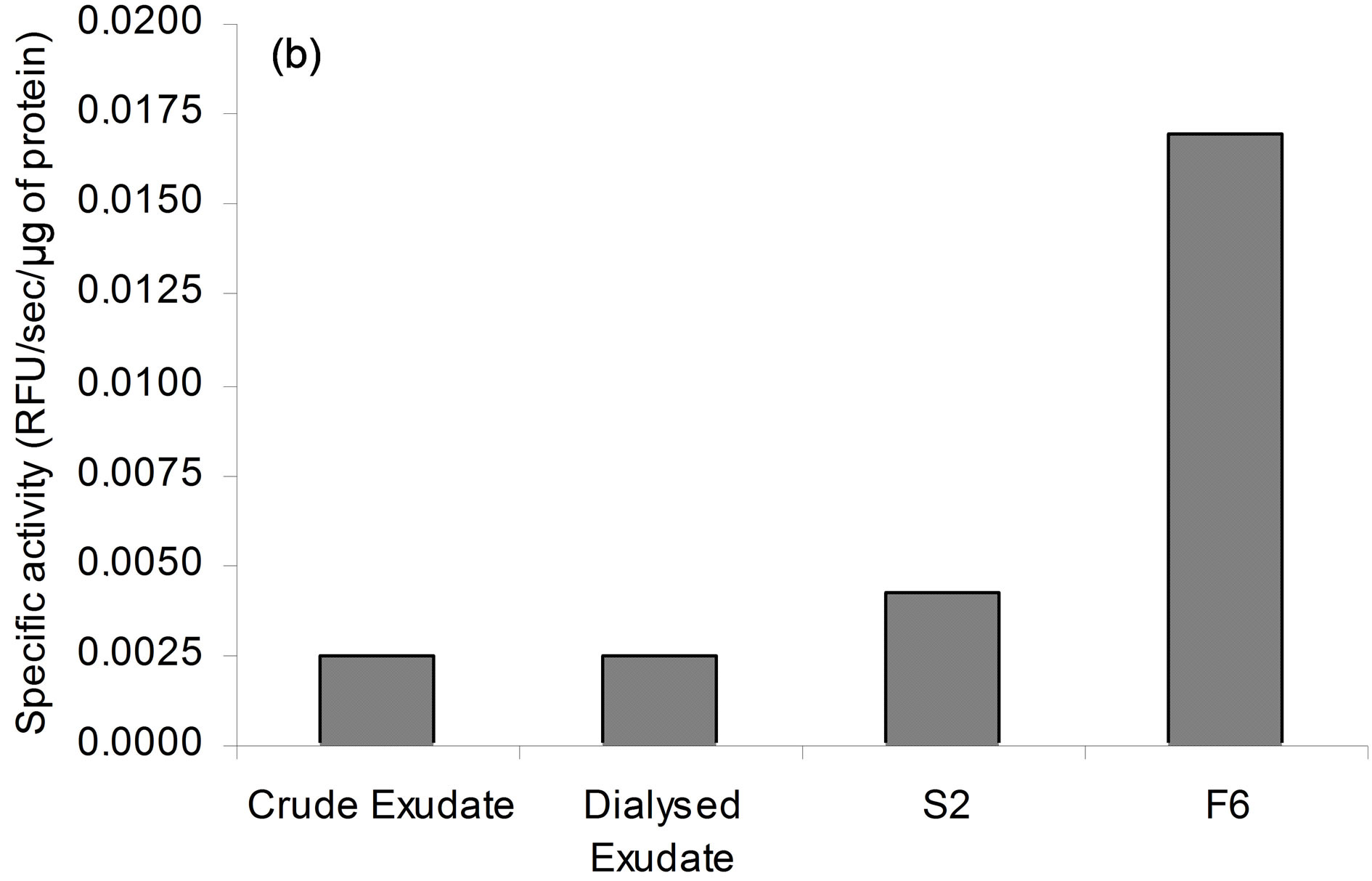
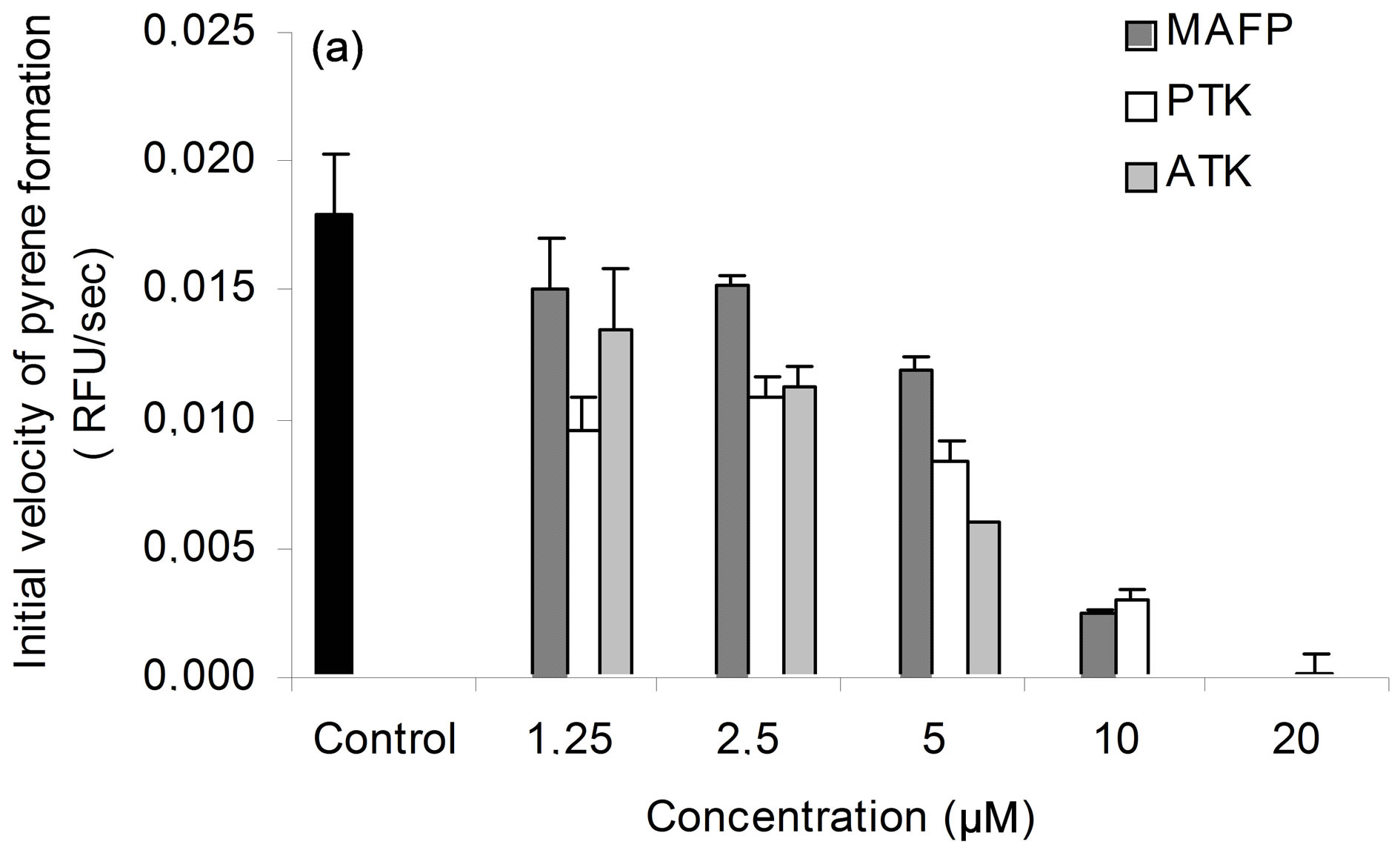
Figure 3. PLA2 activity of purified secreted patatin. (a) Purification of secreted PLA2 following enzyme activity. Each extract corresponds to protocol described in experimental procedure. PLA2 specific activity is given for each step of purification. Results are representative of five independent experiments. (b) Sensitivity of secreted PLA2 activity to specific PLA2 inhibitors. Fraction S2 of secreted patatin-like PLA2 was mixed with 0.01% (v/v) DMSO (control; black histogram) or MAFP (striped), PTK (white) or ATK (grey), at indicated concentrations. Fraction S2 was a purified secreted product that was 4-fold concentrated prior to use. These results are representative of three independent experiments.

Figure 4. Effect of a purified fraction on plant tumour cells. Crown-gall tissue was obtained on a piece of H. tuberosus tuber parenchyma. Fragments of tumour tissue were put either into distilled water (Petri plate A) or in a Petri plate containing 40 kDa polypeptides (Fraction 6 from sephacrylamide column; Petri plate B). Tumour tissues were observed two days later. Arrows show areas of necrotic tissues.
 (a)
(a)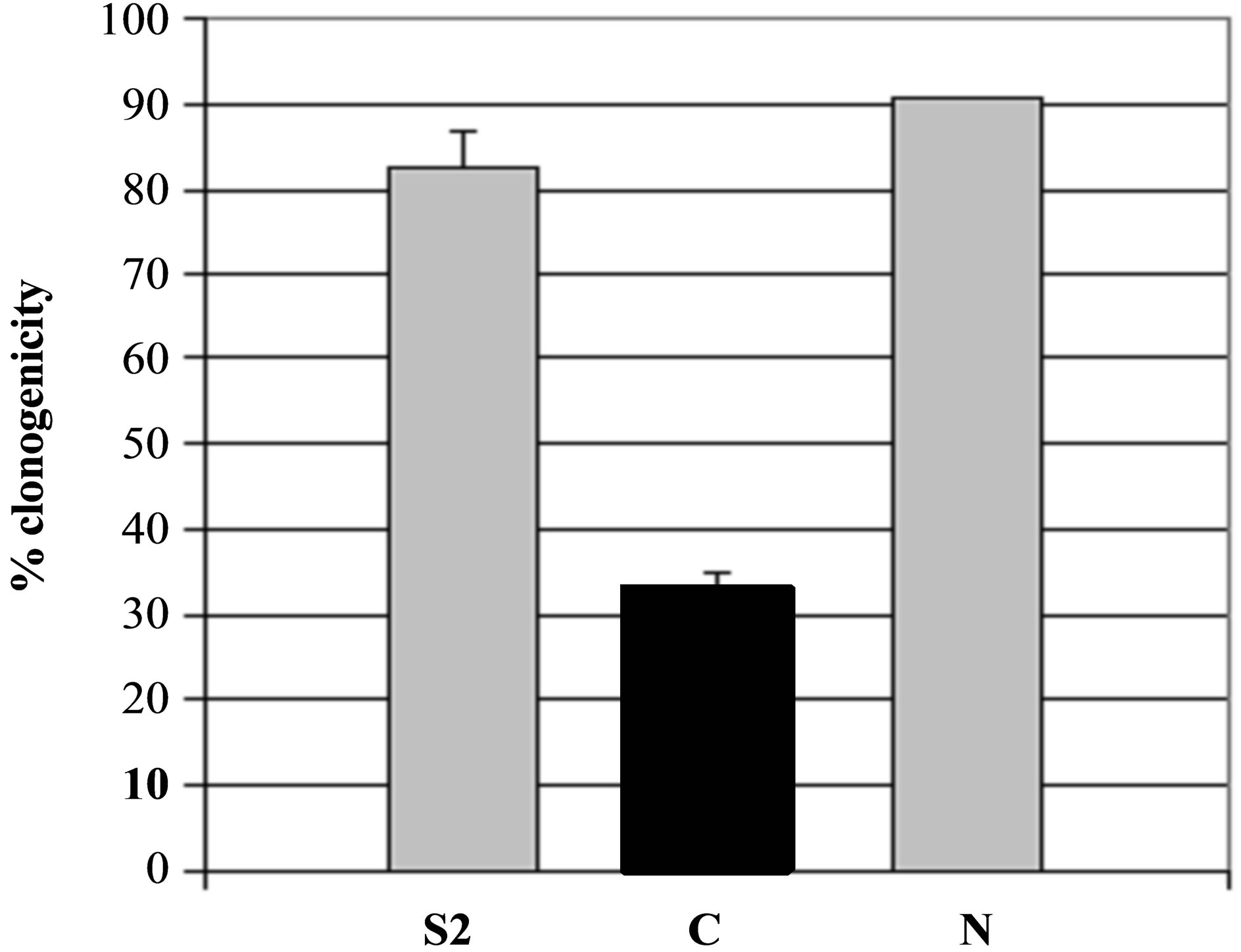 (b)
(b)
Figure 5. Specificity of secreted patatin polypeptide activity against B16 melanoma cells. (a) PLA2 activity of different kind of secreted fraction, such as fraction S2, patatin-like protein fraction retained on Concanavalin A column (C) and fraction not retained on column (N) that was shown to be deprived of patatin-like polypeptides. PLA2 specific activity is given as 10−6 RFU/sec/µg protein. (b) Clonogenicity of B16 cells after treatment with similar concentrations of protein in comparison with control cell cultures.
3.4. Cytotoxicity on Murine Tumour Cells
To evaluate secreted product as potential agent against mammal tumours, its cytotoxicity was investigated in vitro by colony-forming assay both on murine B16 melanoma cells and on murine L929 non-tumour cells (Figure 5(b)). Cytotoxicity against B16 cells was correlated to increased patatin-like polypeptide purified by concanavalin A (Figure 5(b)), compared with crude product (S2). In contrast, fraction of the product that was not retained on column (and thus depleted for 40- kDa-protein), had no significant PLA2 activity (Figure 5(a), lane N) and displayed a reduced cytotoxicity against B16 cells (Figure 5(b), lane N).
Although purified fraction F6 showed no cytotoxicity on L929 fibroblasts in the same range of concentrations, a dramatic decrease of clonogenicity was observed for B16 melanoma cells treated with either secreted product (data not shown) or purified fraction F6 (Figure 6). Cytotoxic activity was thus concentration-dependent and purified fraction F6 showed an IC50 of approximately 0.42 µg protein ml−1 on B16 cells.
3.5. PLA2 Activity Inhibition and Cytotoxicity
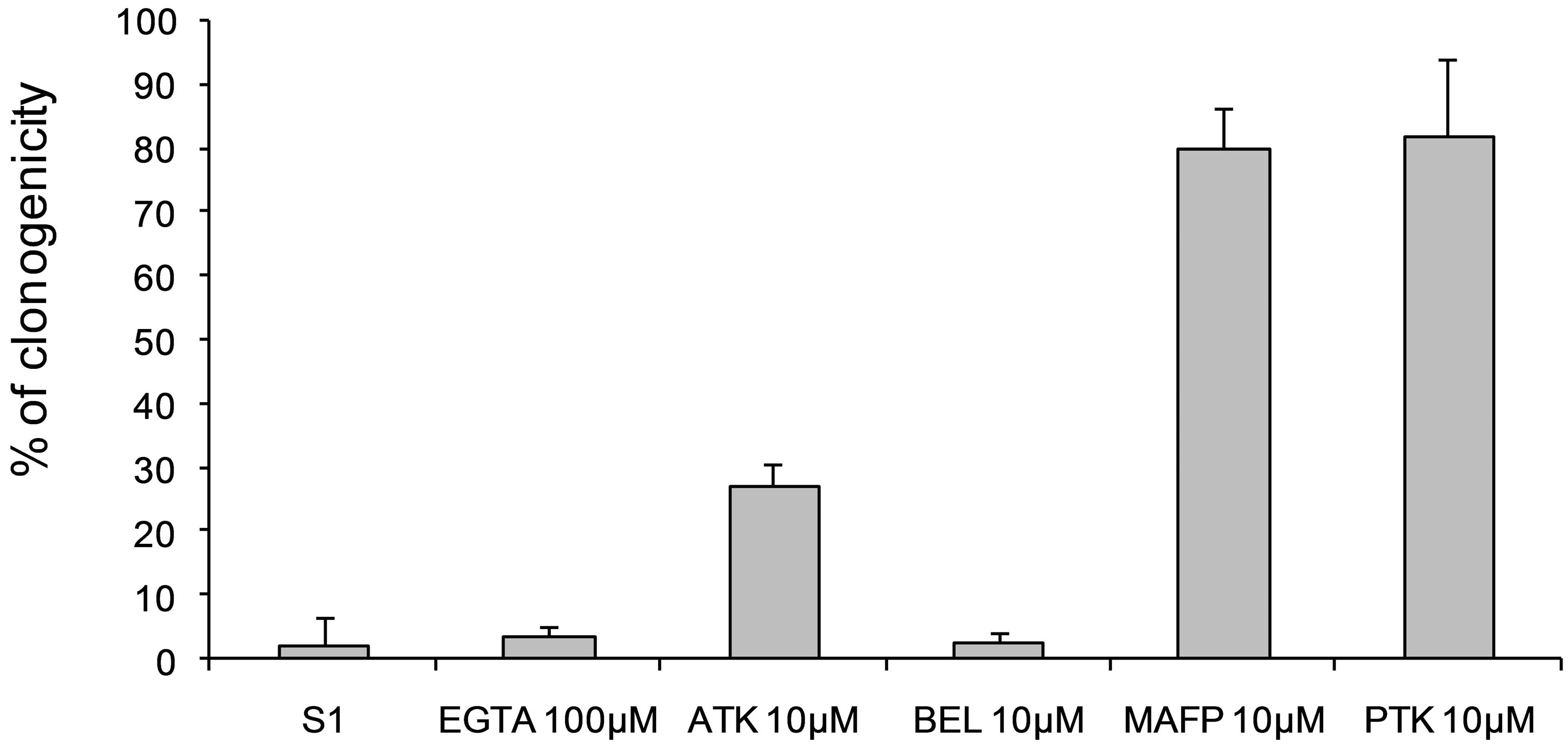
MAFP and PTK inhibitors at 10 µM decreased cytotoxic activity of secreted protein against B16 cells, while ATK had an intermediate effect and BEL did not show any effect (Figure 7). Moreover, addition of EGTA (100 µM), a calcium chelator, did not modify cytotoxicity of secreted product. Therefore, PLA2 activity described above is at least in part involved in a specific cytotoxicity of secreted proteins against B16 melanoma cells.
4. DISCUSSION
Following a water stress, wounded potato tuber parenchyma secreted products including several proteins during rehydration period that were toxic against tumour cells in vitro. Potato tubers cut into pieces and air-dried in sterile tubes displayed a scar tissue as described by Baji et al. [41]. Active agent, which involved one 36-40 kDa protein, or possibly a protein complex, is related to patatin-like PLA2. It induced specific cytotoxicity against plant crown-gall cells and against murine B16 melanoma cells. Two or four cycles of desiccation-rehydration also produced secretion of cytotoxic agent. In addition, sodium orthovanadate, commonly used to inhibit ATPbinding cassette transporters [36], prevented secretion of patatin-like polypeptide. It is likely that an ATP-dependent cellular process is involved in 40 kDa fraction secretion. This may suggest that secretion is involved in and as-yet undescribed plant defence system activated by a stress.
Main protein was purified from plant secreted product
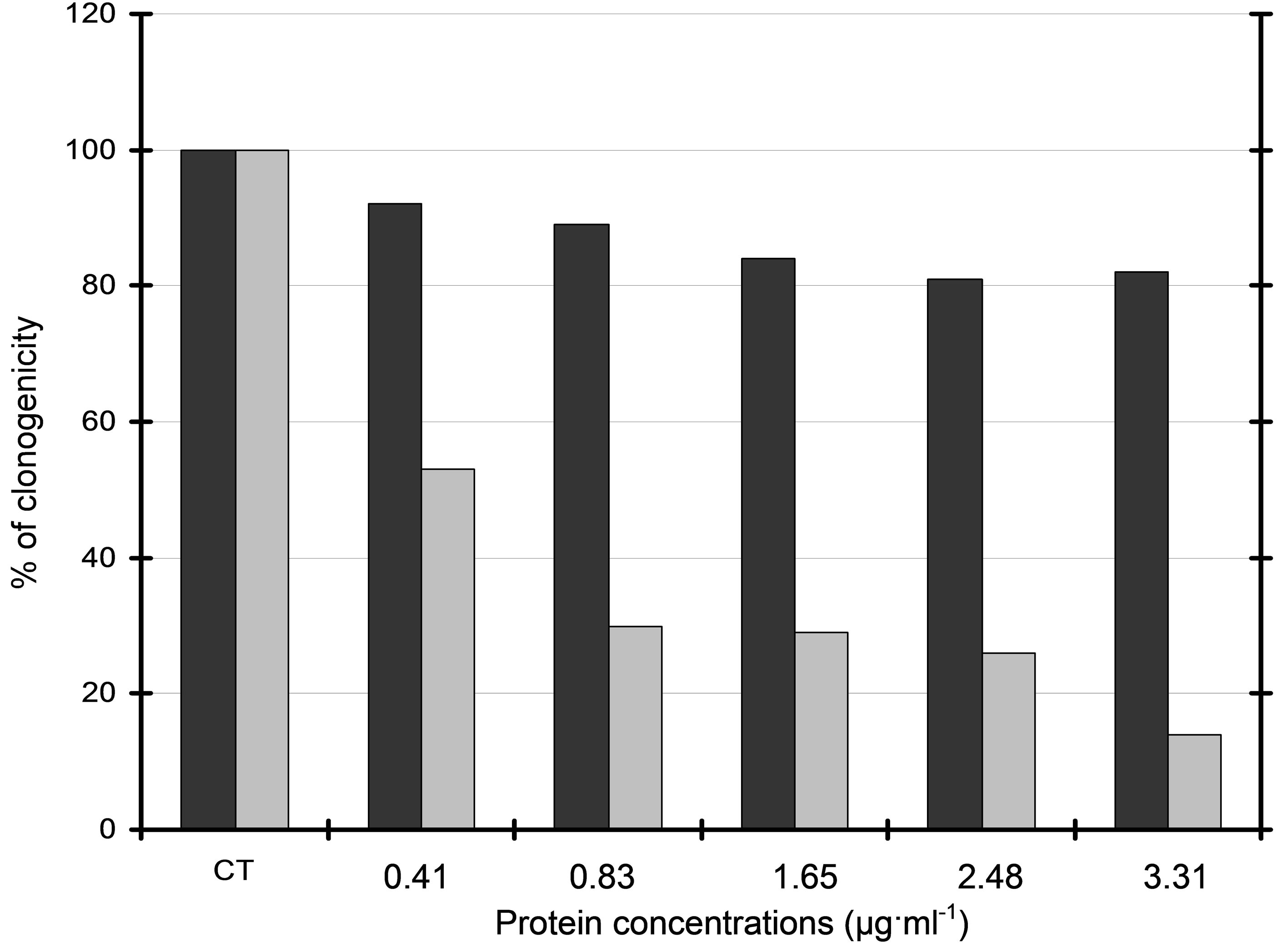
Figure 6. Clonogenicity of B16 melanoma cells treated with purified fraction 6. Concentration-dependent cytoxicity of sephacrylamide-separated fraction 6 was investigated using a colony forming assay of B16 cells (grey histograms) and L929 immortalized fibroblasts (black histograms). Clonogenicity was expressed as % of control cells CT (untreated B16 and L929 cells respectively). Observed IC50 was 0.42 µg∙ml−1.
Treatment
Figure 7. Effects of PLA2 inhibitors (EGTA 100 µM, ATK 10 µM, BEL 10 µM, PTK 10 µM, 2,4D 10 µM) associated with secreted patatin-like protein treatment on B16 cell viability. Fraction S2 was a purified secretion product that was 4-fold concentrated prior to use; each experimental sample contained 3.5 µg of secreted patatin-like protein.
through sephacrylamide column and was shown to belong to patatin family. Secreted patatin-like product could be recovered from plant product by concanavalin A lectin engrafted column, as described for intra-cellular patatin [37]. SDS-PAGE pattern of patatin-like protein retained on concanavalin A column, showed a pattern similar to the fraction 6 polypeptides separated on sephacrylamide column (Figure 3), and have been used for enzyme and cytoxicity assays with similar results. In plants, patatin is mainly observed in vacuoles of tuber cells, and sometimes in cytosol. However, it was shown that treatment of potato tubers by elicitors such as jasmonic acid, salicylic acid or arachidonic acid, promotes excretion of patatin and chymotrypsin inhibitors [38].
Amino terminal sequence of secreted patatin-like polypeptides displayed a nucleotide-binding consensus motif GGGIKG previously described in mammalian iPLA2α sequence (40 kDa, 386 amino acids) [34] (Table 1). This sequence was localised 12 amino acids aside from N-terminal end of secreted patatin-like sequence, versus 36 amino acids from N-terminus in mammal iPLA2. The sequence SIDGGG, which is displayed, was previously described in human iPLA2α sequence [39].
Patatin-like proteins from several plant sources have been reported to exhibit PLA2 activity [40]. Structural analysis of plant lipid acylhydrolase patatin revealed that it also contained a Ser/Asp catalytic dyad and an active site structurally similar to cPLA2α [14]. Patatin-like PLA2 size in animals and plants ranges from 40 to 48 kDa, and purified enzyme from broad beans, was inhibited by mammalian iPLA2 and cPLA2 inhibitors [42]. Our work has shown that secreted patatin-like protein complex displays PLA2 enzyme activity, but with special features. Addition of ATK, PTK, and to a lesser extent MAFP, which are well-known inhibitors of cPLA2 and iPLA2 activities, decreased enzyme activity of secreted patatin-like protein. In contrast, BEL, which specifically inhibits iPLA2 activity, did not significantly decrease lipid acyl hydrolase activity of the product, and addition of EGTA, a calcium chelator which inhibits cPLA2 activity, also did not decrease acyl hydrolase activity. Therefore, secreted patatin-like protein displayed unusual features for PLA2 activity, and could be classed as BEL-insensitive iPLA2.
Sensitivity of lipolytic enzymes to different chemical inhibitors can also be used to discriminate active sites in subfamilies. For example, pPLA and iPLA2 share a sensitivity against BEL inhibitor (also called HELSS), showing again their close catalytic properties. Regulation of cell death is also inhibited by BEL so that the relevant protein was shown to have characteristics of a pPLA [4,43].
Altered lipid biosynthesis and deregulated lipogenesis are typical features of cancer [44]. We showed that PLA2 activity was required for cytotoxic effect on B16 melanoma cells. It induced cell death of crown-gall and inhibited clonogenicity of B16 cells, but, at same concentration, was not toxic for non-transformed cells. Inhibition of B16 cell clonogenicity was correlated to increase of PLA2 activity in fraction used for treatment (Figure 5) and cytotoxicity was shown to be sensitive to same PLA2 inhibitors. Moreover, secreted product cytotoxicity was lost after protein precipitation with ammonium sulphate or when 40 kDa polypeptide group was depleted. These data are in agreement with direct cytotoxic effect of PLA2 activity of 40 kDa patatin-like polypeptide. Otherwise, cell-stored patatin of plants, a form of cPLA2, and sPLA2 from naja were ineffective against plant tumour and melanoma B16 cells (data not shown).
Although PLA2 biochemistry is well documented, its mechanism of tumor cell cytotoxicity is still the subject of much debate. Several studies have shown correlation between epidermal growth factor receptor (EGFR) expression and sensitivity to PLA2 [45,46]. However, both stimulatory and inhibitory effects have been reported, perhaps due to PLA2 and cell type and/or concentration effects. SPLA2 has been explored as an anticancer agent in its own right [47] as the active component of nanosized drug delivery systems [44,45]. Lei et al. [48] suggest that iPLA2β activates a unique signaling cascade that promotes β-cell apoptosis. These observations indicate that p38 MAPK is activated downstream of iPLA2β in β-cells and that p38 MAPK is involved in the β-cell functional responses of insulin secretion and apoptosis in which iPLA2β participates [49]. Otherwise, Protobothrops flavoviridis venom contains plural phospholipase A2 isozymes that induced apoptotic cell death, neither inhibited by inhibitors of caspases 3 and 6 nor accompanied by activation of procaspase 3, indicating that BPII-induced cell death was caspase independent, and mediated via cell-surface receptor [50]. Moreover, the inhibitory action of viper sPLA(2)s towards cancer cells depended on both venom and cell type. VBBPLA2 (Vipera berus berus) inhibited significantly the viability of K-562 cancer cells and the cell death appeared apoptotic. The sPLA2s exhibited no inhibitory effect towards LNCaP cancer cells and some effect towards other cells [51].
Recently, an extracellular multifunctional protein, PEDF, belonging to the serpin superfamily with a demonstrable antitumorigenic property, was shown to have a high affinity for the plasma membrane protein PEDF-R, which displays a phospholipase A2 activity [52]. The authors suggested that PEDF-R may produce extracellular bioactive lipids, which can diffuse back into the cell as signalling molecules. PEDF, by binding to membrane-localized PEDF-R, may stimulate release of specific fatty acids and lysophospholipids, irreversibly damaging tumour cells [52,53].
iPLA2 is in common use for animal enzymes. iPLA2 induced apoptosis in U937 human monocytic leukaemia cells [34]. This effect is associated with hydrolysis of arachidonic acid from membrane phospholipids through a mechanism inhibited by BEL, and was not catalysed by sPLA2 or by cPLA2. Arachidonic acid may serve to activate caspases and other downstream apoptotic signalling pathways [54]. Patatin-related proteins in plants, animal iPLA2s and many bacterial proteins are characterized by having a catalytic center consisting of esterase box GTSTG and phosphateor anion-binding element DGGGXRG [22]. These sequence elements are also present in iPLA2α, iPLA2β and iPLA2γ [4,40].
Extracellular and intracellular PLA2 isozymes have been implicated in several types of apoptosis [55-57]. Antitumour factors such as TNFβ trigger PLA2 activity in macrophages, similar in molecular weight and PLA2 inhibitor sensitivity to that described here [55]. Inhibition of iPLA2 protected against chemotherapeutic-induced cell death in human renal cell models, and identified specific phospholipids whose levels are altered during cell death [58]. Activation of phospholipase A2 was shown potently to induce apoptosis [59].
Aside from its enzymatic activity, secreted patatin-like protein may also influence tumour growth through its ability to act as a ligand for M-type receptor, thereby activating downstream signalling pathways that influence proliferation or apoptosis, as described for sPLA2-IIA [60,61].
We have shown a specific activity against tumour cells. One of the main differences between tumour and healthy animal cells lies in the fact that their lipid compositions and distributions are different. In normal conditions, membrane phospholipids are asymmetrically distributed across cell membrane monolayers, with phosphatidylserine (PS) and phosphatidylethanolamine (PE) located on inner face, and sphingomyeline (SM) and phosphatedylcholine (PC) on outer face [62,63]. Asymmetric distribution of lipids was also observed in plasma membrane of tumour cells, but with reversed localization for phosphatidylserine (PS), which is present on outer face [64]. Remarkably, numerous tumour cell lines have been reported to display 3- to 7-fold elevated amounts of PS on exoplasmic membrane leaflet compared with nontumorigenic cells [65]. Therefore, we can suggest that different patatin-like proteins, and especially secreted patatin, may have different substrate preferences, as indicated by earlier results for other patatin-like products [66]. Thus, iPLA2-like enzyme (secreted patatin-like protein, e-patatin), whose secretion from potato tubers is triggered by physical stress, may recognize and mainly bind anionic phospholipids PS or PE, localized on external leaflet of tumour cell membrane. It was shown that bee venom PLA2 membrane interaction was mediated by a domain mainly composed by hydrophobic residues and two basic residues promoting interaction with anionic phospholipids such as phosphatidylserine (PS) [44]. Thus, both electrostatic and hydrophobic interactions determine location of PLA2 relative to membrane bilayer [67,68].
PLA2 activity of secreted patatin-like protein would hydrolyse phospholipids into an inducer of apoptosis or necrosis, such as free arachidonic acid or specific lysophosphatidic acid. It was shown that arachidonic acid administration caused apoptosis in Y79 cells, as shown by typical morphological changes, phosphatidylserine externalization, chromatin condensation, processing and activation of caspase-3 and cleavage of the endogenous caspase substrate poly-(ADP-ribose)-polymerase [59]. Arachidonic acid itself could induce apoptosis in cultured cells. These observations provide evidence that arachidonate may be involved in apoptosis in animal cells [69,70]. Arachidonic acid treatment was accompanied by increased formation of the lipid peroxidation end products malondialdehyde and 4-hydroxy-2-nonenal [59]. Arachidonic acid (AA) was described as lipid messenger derived through cleavage of membrane phospholipids by phospholipase A2 (PLA2) [71]. Exogenous arachidonic acid caused apoptosis in colon cancer and other cell lines [72]. One is to use phospholipase A2 (PLA2) to cleave phospholipid heads of the bilayer of cancer cells [73]. Correlative and functional studies involved expression of some secreted enzymes (sPLA2s), particularly the best studied enzyme Group IIA sPLA2 in either tumour promotion or inhibition, depending on organ involved and biochemical microenvironment of tumours [74].
Apoptosis in HL-60 cells, induced by blocking arachidonate-phospholipid remodeling, is correlated with redistribution of arachidonate in membrane phospholipids and suggest that such alterations represent a signal which controls capacity of cells to proliferate [75]. Arachidonic acid (AA) is not only a major structural constituent of membranes and a key precursor of various bioactive molecules (eicosanoids in particular), but it is also a second intracellular messenger. Its release from membrane phospholipids occurs upon several hormonal stimuli and in the course of pathological situations, ischemia injury in particular. This mainly relies on the activation of phospholipases A2 (PLA2), but may also involve phospholipases C or D, and results in an increase in intracellular level of unesterified AA and is a good candidate as mediator of apoptosis triggered by TNFalpha, a role of AA which has been formally established in human leukemia cells [76]. Arachidonic acid released by Phospholipase A2 activation triggers Ca2+-dependent apoptosis through mitochondrial pathway [77].
Mitochondrial COX-2 in cancer cells confers resistance to apoptosis by reducing the proapoptotic arachidonic acid [78]. Natural PLA2 activity, as well as each subtype of PLA2 activity was elevated in each cancer group as compared to healthy controls. Phospholipase A2 enzymes (PLA2s) are major enzymes producing cyclooxygenase-2 (COX-2) substrate, arachidonic acid (AA), as well as lysophospholipids. Both of these classes of products are signaling molecules involved in cancers. More importantly, their enzymatic activities, but not necessarily their RNA and/or protein expression levels, are directly related to biological effects, since PLA2 activities are well-known to be regulated post-transcriptionally [79-81]. Cytotoxicity of arachidonic acid appeared to involve lipid peroxidation mechanism. Arachidonic acid-induced apoptosis of human neuroblastoma SK-N-SH cells is mediated through mitochondrial alteration elicited by ROS and Ca2+-evoked activation of p38α MAPK and JNK1 [82]. It is notable that lipoapoptosis, induced by palmitic acid in human hepatocytes, was dependent on PLA2-generated lysophosphatidylcholine (LPC). It could be blocked by PLA2 inhibitors. Since, in plants, strong PLA2 induction precedes appearance of hypersensitive cell death, and various PCD types could either be accelerated or postponed by PLA2 overexpression or suppression, respectively, it can be proposed that PLA2 exerts its function in plant defense responses through the acyl remodeling of phospholipids [83]. Cell cytotoxic effects through activation of PLA2 by melittin have been suggested to be critical mechanism for anti-cancer activity of bee venom. Apoptotic cell death induction through several cancer cell death mechanisms, including activation of caspase and matrix metalloproteinases, is important for melittin-induced anti-cancer effects [84]. Loss of cPLA2 activity, either through genetic knockout in mice, or by treatment with a cPLA2 inhibitor, results in an attenuation of arachidonic acid release as well as of the apoptotic response to oxLDL in peritoneal macrophages or to 25-OHC in cultured fibroblast and macrophage cell lines [85].
We showed that effect of secreted patatin on tumour cells mainly proceeds by apoptosis. Necrosis would process through production of lysophospholipids, which can decrease resistance of cell membrane. Lysophospholipids, lysoPA, are lipid mediators that are involved in wide variety of biological responses in animals. Recently, IIA PLA2 protein content was shown dramatically decreased in malignant colorectal tumours as compared with adenomas. The protein was also found in apoptotic cells, necrosis, peritumoural mucosa and in invasive front of carcinomas [86].
Drugs targeted cancer cell membrane might become a new and high effective clinical cancer therapy. One is to use phospholipase A2 (PLA2) to cleave phospholipid heads of the bilayer of cancer cells [73]. Degradative action of LAH may alter capacity of lipid bilayer to regulate exchanges between compartments, but many subsequent physiological responses are due to novel biological properties of lysolipids or oxygenated fatty acid derivatives that are generated [87]. Arabidopsis Patatin-Like Protein 2 (PLP2) plays an essential role in cell death execution and differentially affects biosynthesis of oxylipins and resistance to pathogens. PLP2 is an integral component of the plant cell death execution machinery, possibly providing fatty acid precursors for the biosynthesis of specific oxylipins and differentially affecting resistance to pathogens with distinct lifestyles [22].
Among the large family of PLA2 enzymes, calciumindependent phospholipases A2 (iPLA2) represent a relatively novel subgroup. The fact that iPLA2 have been recently described as key mediators for proliferation of cancer cells, such as colon cancer, ovarian cancer or prostate cancer cells, emphasizes their potential role as molecular targets for cancer treatment [88,89]. Functional role of iPLA2 in prostate cancer has been mainly demonstrated in vitro. Recently, down-regulation of iPLA2-β in prostate tumor cells has been correlated with reduced tumorigenesis and metastasis in vivo [90,91].
A model for specific activity of secreted patatin (e-patatin) on tumour cells can be proposed (Figure 8). We suggest that iPLA2-like enzyme (e-patatin), which can be obtained from secretion of potato tubers triggered by a physical stress, recognizes and mainly binds anionic phospholipids PS or PE, localized on external leaflet of tumour cell membrane. Subsequently, arachidonic acid, in animal cells, or linonenic acid, in plant cells, is released which, in turn, induces apoptotic pathway or may trigger necrosis. Then, enhanced free PUFA would increase the activity of iPLA2 enzyme, as it was previously described for activation of sPLA2 [92]. We have also observed that mixture of linoleic acid and purified e-patatin showed higher cytotoxic effect on crown-gall cells than e-patatin alone; at this concentration, linoleic acid alone has no effect on plant tumour cells.
Previously, we have shown that wound-desiccation stress on various storage plant tissues allowed secretion of products that had specific cytotoxicity against melanoma B16 cells. Healthy plant or animal cells were not susceptible to these products. Secretion of active product varied with plant species and plant organs. Secretion products of tubers of yam (Dioscorea cayenensis Lam), cassava (Manihot esculenta), Jerusalem artichoke (Helianthus tuberosus), ginger (Zingiber officinale) and potato (Solanum tuberosum L.) showed activity against murine melanoma B16 cells, but not against immortalized fibroblast L929 cells. Active agent was associated with a protein complex. Secretion of cytotoxic products against melanoma B16 cells, thus, can be performed through specific stress on storage organs of plants usually cultivated [93]. In Jerusalem artichoke (Helianthus tuberosus L.) tubers, active agent was shown to contain 18-kDa polypeptide with homology to superoxide dismutase (SOD) [2].
5. CONCLUSION
In summary, these findings support a model put forward in Figure 8 where iPLA2-patatin reveals as a protein with a multifunctional role. Therefore, a double stress of potato tubers induces secretion of a patatin-like protein that displays an unusual iPLA2 enzyme activity, which is required for its specific activity against B16 tumour cells. The prevention and therapy of cancer may benefit from the introduction of new treatments derived from natural products. Many pharmaceutical products approved for

Figure 8. Model for secretion product, e-patatin, effect against tumour cells. Lipid messengers derived from hydrolysis of plasma membrane [94]. AA: arachidonic acid; LA: linoleic acid; LysoP: lysophospholipids. PE: phosphatidylethanolamine; PS: phosphatidylserine; Arrows indicate flow of signalling pathway. Secreted e-patatin/iPLA2, from safe plant cells, directly acts on outer leaflet of tumour cell membrane to release fatty acids (including arachidonic acid or linolenic acid) and lysophospholipids, both of which can activate signal transduction pathway (apoptosis), by increasing Ca2+ concentration. Eventually, a pathway triggering the weakness of the cell membrane (necrosis) can be developed.
human disease treatment are derived from natural sources [95]. This patatin-like PLA2 thus represents a new member of the lipolytic superfamily and could represent a novel therapeutic product for the development of new anticancer agents.
6. ACKNOWLEDGEMENTS
This research was supported by grants from ANVAR (n° A9907080C/ AT) and ARC. We are grateful to Pr. M. Andréani, Dr. N. Uhrhammer, Dr. J. C. Maurizis for their skilful advices, to R. Cochet, N. Roussel, G. Evray, D. Marcon, and C. Vian for their skilful technical assistance.
ABBREVIATIONS
AA = arachidonic acid ATK = Arachidonyl trifluoromethyl ketone BEL = bromoenol lactone, E)-6-(bromomethylene)-3- (1-naphtalenyl)2H-tetrahydropyran-2-one cPLA2 = cytosolic phospholipase A2
2D IEF = Two-dimension isoelectric focusing DMSO = dimethylsulfoxide 2D PAGE = Two-dimension Polyacrylamide Gel Electrophoresis iPLA2 = calcium-independent phospholipase A2
MAFP = methyl arachidonyl fluoromethyl phosphonate MW = molecular weight PC = phosphatidylcholine PE = phosphatidylethanolamine PLA2 = phospholipase A2
PR = Pathogenesis-Related PS = phosphatidylserine PTK = Palmitoyl trifluorylmethyl ketone PUFA = Polyunsaturated Fatty Acid SDS PAGE = Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis SM = sphingomyeline
NOTES