Preparation and Characterization of Conductive Cellulosic Fabric by Polymerization of Pyrrole ()
1. Introduction
Electronic textiles are fabrics/garments that contain electronic circuits, optical fibers or sensors. Such functional textiles provide potential opportunities for boundless applications in electronic interfaces [1] and in the field of health care. It is highly likely that within 10 years, computer chips will be integrated into garments and wearable technology will become as common as mobile phones. One of the most practical techniques to make textiles electrically conductive is to apply conductive polymers to the fabric surface. Conductive polymers have a comparable degradation lifetime to textile materials [2]. The coating is easily applied and does not have a significant effect on the fabric softness. Therefore, conductive polymer coated textiles are promising materials for wearable electronics.
Conductive polymers have attracted the attention of a great number of researchers in the textile field due to their potential applications in composites with natural, artificial or synthetic fibers. The affinity to several kinds of fibers, yarns and fabrics with doped conjugated polymers, permits the production of composite textiles with improved electrical properties. Polypyrrole (Ppyr) is one of the most suitable conductive polymers for deposition on textile materials due to its excellent conductivity and relevant environmental stability [3-5]. Ppyr is commonly produced by electrochemical synthesis or chemical oxidative polymerization in aqueous solution and shows good affinity with natural and artificial fibers. Textile substrates can be easily covered with a Ppyr layer by immersion of the fabric in the polymerization solution containing pyrrole (Pyr), an oxidant and a doping agent [6-8].
In the wake, the interest shown by the microelectronics industry for intrinsic conducting polymers (ICPs) which is from the textile, and paper industries for the wide range of new functionalities of ICPs have increased progressively during the last two decades. Conductive fabrics from natural (wool and cellulose) [9,10] and synthetic [11] fibers have been elaborated by polymerizing in situ pyrrole using ferric compounds as oxidizing agents.
Recent research shows that polypyrrole can be embedded in natural and manmade cellulose based fibers, such as cotton, viscose, cupro and lyocell, by means of internal polymerization taking place in the amorphous region of the fibers [12]. Pyrrole was applied by a simple finishing process at room temperature from an aqueous solution of the monomer that penetrates into the cellulose substrate similarly to a non-reactive dye. Subsequent polymerization caused its permanent insolubility in the internal fiber structure, leading to high levels of fastness to washing and to light exposure, with significant modification of the mechanical properties of the fibers [13-15]. The type of monomer and the dopant anion, concentrations, and synthesis variables such as time, temperature, agitation, and sequence of exposure to chemicals vary among different research groups, and each of these parameters has an effect on the electrical, morphological and mechanical properties, and the stability of the resulting material [14].
In this work, the chemical oxidation polymerization process reaction conditions (monomer concentration, catalyst: dopant ratio and temperature) have been optimized in order to obtain the best properties of the treated fabric. We report the effects of coating cotton fabric by conductive polypyrrole films on electrical properties, color strength, weight gain, tensile strength and elongation.
2. Experimental and Methods
2.1. Fabrics and Materials
White cotton fabric was obtained from INOTEX Company, Czech Republic, Pyrrole monomer from Merck. Ferric chloride of analytical reagent grade from BDH Chemicals was used. Tetra-ethylammonium p-Toluenesulfonate [(C2H5)4N(CH3C6H4SO3)] was obtained from Aldrich Chemicals. All other chemicals used were of analytical grade. All aqueous solutions were prepared with distilled water.
2.2. Methods
2.2.1. Preparation of Polypyrrole Coated Fabric
Preparation of polypyrole coated fabric obtained by two steps as follows:
1) A piece of fabric was soaked in an aqueous solution containing different amounts of both an oxidant (Ferric Chloride) and dopant (TEAp-TS) for about 20 minutes with fixed ratio between them [2:1 (M/M)] and a fabric/ bath ratio of 1:25, then the substrate was pressed with an automatic padder with considering in mind that the wet pick-up (the weight of the liquid as the percentage weight of the substrates) is about 100%.
2) The treated sample with catalyst and dopant was allowed to soak in the Ahiba Nuance dye pots contain a solution of pyrrole in water ,the pots were placed in a rapid rotary dyer. The polymerization was carried out for 4 hours at 25˚C with a fabric/bath ratio of 1:25 using different monomer concentrations (0.1 M to 0.4 M). Agitation was supplied via rotation of the pots at 25 revolutions per minute. Rotation direction was changed every 1 minute. After polymerization the samples were rinsed with water to remove the polymer excess and the samples were dried at ambient conditions. The fabric was black in color resembling polypyrrole.
2.3. Testing and Analysis
2.3.1. Weight Gain Percent
The amount of PPy deposited on the cotton fabrics was determined by weighing the cotton samples before and after treatment under standard conditions of temperature (20˚C) and relative humidity (65%). The percentage weight increase (W%) was calculated as follows:

where Wi and Wf are the initial and final weight, respectively.
2.3.2. Characterization of Electrical Properties
The conductivity of the PPy-coated cotton fabric was measured using the AATCC 76 two point-probe technique using FLUKA digital multimeter. The bulk resistances of the dried and as prepared samples were measured at room temperature using Professional Digital Multimeter (uni-T, UT70C). The sample conductivity, σ, was obtained from the measured resistance R according to σ = (1/R).
2.3.3. Tensile Strength
Fabric tensile strength test was conducted according to ASTM method 1682 (1994) (using Testometric (M350- 5CT), which is a standard method for breaking force and elongation of tensile fabrics [16]. The width and the length of the fabric strip were 50 mm and 200 mm respectively.
2.3.4. Color Measurement
The color intensity expressed as K/S value, of the stained samples before and after treatment was, as a function for polymerization efficiency, determined spectrophotometrically using Datacolor spectrophotometer. K/S was calculated by applying the Kubelka-Munk equation [17].
All the determinations in this work were done in triplicate and the results present mean values.
2.3.5. Scanning Electron Microscopy (SEM)
The surface morphology of untreated and treated fabric was investigated by using SEM, VEGA (TESCAN, Czech Republic). Before examination, the fabric surface was prepared on an appropriate disk and coated randomly by a spray of gold.
2.3.6. FT-IR Spectroscopy
The FT-IR spectra of cotton fabrics treated with polypyrrole were recorded on a FT-IR spectrometer PerkinElmer, in the spectra range 4000 - 400 cm−1 using the KBr disc technique. All the measurements in this work were carried out in Faculty of Textile Engineering, Liberec, CZ.
3. Results and Discussion
3.1. Tentative Mechanism of the Polymerization Reaction
The reaction is initiated by the oxidation of monomer into radical cations, which combine to form dimers. Continuation of the process leads to formation of insoluble oligomers in solution, which deposit on the surface and interstices of the textile fibers and fabrics. Due to low oxidation potential of pyrrole, a wide range of oxidizing agents can be used to initiate polymerization. Oxidant salts such as ferric chloride (FeCl3) function as both the oxidant and the dopant agent, and hence the polymer is obtained in the conducting form. Other transition metal salts can also be used as oxidizing agents for the polymerization of pyrrole. The transition metal ion is an electron acceptor. Therefore it oxidizes the π-electron system of the pyrrole ring at the initiation step. The intermediate steps of the polymerization of pyrrole initiated by FeCl3 are as follows: The pyrrole molecule is oxidized to yield a radical cation as follows [14]:

Radical cations recombine to form dications:

Deprotonation of dication to yield a dimer:
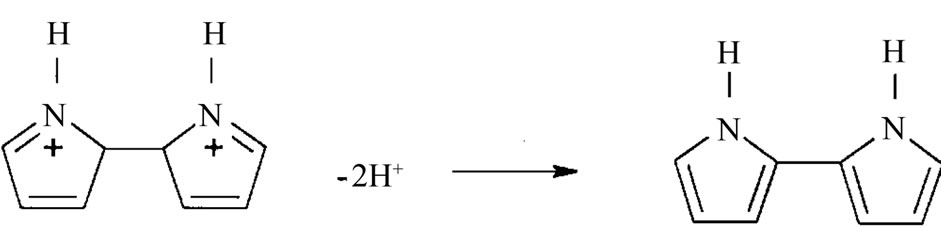
The above process continues by oxidation of the dimer to yield a radical cation of the dimer and the combination of radical cations to form trimers, tetramers, and polymers. Sulfonate anion also attaches itself to pyrrole molecule during chemical polymerization. Oxidation of polypyrrole results in a partial positive charge on the pyrrole ring, which attracts anionic species such as p-toluene sulfonate in order to achieve charge neutrality as follows [14]:

3.2. Influence of the Ratio between FeCl3 and TEAp-TS
The effect of FeCl3 concentration in admixtures with TEAp-TS on the fabric properties, expressed as weight gain, resistivity, color strength, tensile strength and antimicrobial properties when the treatment was carried out with pyrrole (0.2 M/L) using FeCl3 as an oxidant and TEAp-TS as a dopant, which in turn caused a reduction in the moisture regain of the substrate, at 25˚C for 4 hours are shown in Figures 1-5. It can be seen (Figure 1) that, the use of FeCl3/TEAp-TS mixture causes increasing of fabric properties. When the ratio between FeCl3 and TEAp-TS in the mixture increases, weight gain increases. This is due to the increase of radical cations number which combined with each others to form polymer, this is arises from FeCl3 acted as oxidizing agent of pyrrole for the polymerization of pyrrole on the fabric surface in presence of TEAp-TS as a doping agent and hence quantity of the polymer on the fabric increased.
Constant parameters: [pyrrole]: 0.2 M/L; polymerization time: 4 h; polymerization temperature: 25˚C.
Figures 2 and 3 depict that, tensile strength and elongation of the treated fabric increasing by increasing the ratio between FeCl3 and TEAp-TS in the mixture. The increase in tensile strength and elongation of the coated fabric with polypyrrole was attributed to the reinforcing effect of the high strength and modulus conductive polymer coating on the fiber.
Constant parameters: [pyrrole]: 0.2 M/L; polymerization time: 4 h; polymerization temperature: 25˚C.
Constant parameters: [pyrrole]: 0.2 M/L; polymerization time: 4 h; polymerization temperature: 25˚C.

Figure 1. Percent weight gain of treated cotton fabrics with the increase in FeCl3 concentration in the impregnation aqueous solution.

Figure 2. Coated fabric elongation change under FeCl3 concentration effect.

Figure 3. Effect of FeCl3 concentration in the impregnation aqueous solution on the strength of treated fabrics.
Polymerization of Pyrrole resulted in black cotton fabrics, meaning that the polymer completely coated the surface of the fibers. When we measured the depth of color (K/S) of the coated fabrics we have seen an increase with

Figure 4. Effect of FeCl3 concentration in the impregnation aqueous solution on the color strength of treated fabrics.

Figure 5. Trend of resistivity of the coated fabric as a function of FeCl3 concentration in the impregnation aqueous solution.
the increase in the ratio between FeCl3 and TEAp-TS in the mixture. Kinetics of depth of color increase is shown in Figure 4.
Constant parameters: [pyrrole]: 0.2 M/L; polymerization time: 4 h; polymerization temperature: 25˚C.
On the other hand, there was a significant decrease in the resistivity of the coated fabric with polypyrrole by increasing the ratio between FeCl3 and TEAp-TS in the mixture. The decrease in the resistivity is arises from conductive polymer layer formation on the fabric surface and increase the amount of polymer on the fabric surface as shown in Figure 5. Current results call for a pad bath formulation consisting of FeCl3 (0.25 M/L) and TEApTS (0.125 M/L) as the most appropriate formulation for effecting polymerizing of pyrrole and anchoring on cotton fabrics.
Constant parameters: [pyrrole]: 0.2 M/L; polymerization time: 4 h; polymerization temperature: 25˚C.
3.3. Influence of the Pyrrole Concentration
Table 1 and Figure 6 show that the effect of the pyrrole concentration on the coated fabric with polypyrrole