D-Dimer Concentrations in Preeclampsia and Normal Pregnancy in Kinshasa ()

1. Introduction
Preeclampsia is a reversible pathology, specific to pregnancy, characterized b the association of arterial hypertension and proteinuria. It can be de novo or complicate 10% of pregnancies with arterial hypertension [1] . According to several authors, preeclampsia is one of the leading causes of maternal and foetal morbidity and mortality [1] [2] [3] . It constitutes a real public health problem in Africa where its prevalence varies from 10% to 40% of pregnant women [4] .
According to several authors, pregnancy, especially preeclampsia and major obstetric syndromes are accompanied by active and intense coagulopathy [3] [5] [6] [7] .
In general, pregnancy thus appears as a pro-thrombotic state in which the risk of disseminated intravascular coagulation (DIC) increases about 5 to 10 times compared to non-pregnant women of the same age. This risk is even great after childbirth [3] [4] [5] [8] . Clinically, this gravid thrombotic micro-angiopathy preferentially localizes at several levels, in particular placental and the organs [9] . Biologically, this gravid micro-angiopathy results in thrombocytopenia of variable severity, high consumption of fibrinogen.
The destruction of a blood clot (degradation of fibrin) leads to the production of specific waste products: d-dimers (DD) [8] [10] . The preeclampsia prothrombotic state exposes to a risk multiplied by 3 to 5 of postpartum phlebitis and pulmonary embolism [2] [5] [6] [7] [9] .
The course of such pregnancies and childbirth can be problematic. Indeed, in these pregnant women, the risk of bleeding increases. In addition, the newborn can sometimes also develop a coagulation problem [6] . It is often recommended to pay particular attention during child birth, in particular to avoid the use of instruments such as the suction cup or to take samples from the scalp of the newborn.
Biological markers of d-dimer (DD) coagulation have been used for a very long time in diagnostic and therapeutic decision-making [10] . Indeed, d-dimers were first proposed in the 1980s as an exclusion test first for deep vein thrombosis and then for pulmonary embolism [2] [9] . Since then, they have been widely studied as a diagnostic tool in several gravid puerperal diseases and integrated into diagnostic strategies in clinically suspected patients. Their serum concentrations have a high negative predictive value for preeclampsia [9] .
D-dimer testing is a quick and non-invasive way to rule out abnormal or excessive coagulation [10] . Schutte and Smulders (2016) recommend not ignoring it during pregnancy because their exaggerated increase is the expression of the severity of disseminated intravascular coagulation in a future mother [11] . However, some authors (Rodger et al., 2014) dispute it [12] .
The d-dimers thus reflect the entire process of clot formation (coagulation) and its lysis [3] [7] [8] . Excess thrombin generation is detected by indirect markers (decrease in ant thrombin, elevation of thrombin-ant thrombin complexes, and increase in platelet turnover) and direct markers (mean increase of 10% in thrombin generation potential). It can be detected and monitored in a delocalized way by the coagulation time and by an elevation of D dimers and a peak in plasmin generation [10] [11] [12] .
Ducloy-Bouthors et al. (2010) demonstrated that fibrinolysis is early and intense [3] .
D-dimers can still be used in pregnant women as a biomarker of preeclampsia, if the average plasma concentration of D-dimers in the pregnant population has not yet been reassessed [11] [12] .
Some studies recommend a gestational age-specific reference interval as the cornerstone of diagnosis of preeclampsia inflammation [13] - [18] .
However, preeclampsia inflammation is silent in its early stages of development and is sometimes a serious disease characterized by multisystem involvement during pregnancy [19] . D-dimer testing is a quick and non-invasive way to rule out abnormal or excessive coagulation. Schutte and Smulders (2016) recommend ͦ not ignoring it during pregnancy because their exaggerated increase is the expression of the severity of disseminated intravascular coagulation in a future mother [11] . However, some authors dispute it [12] [19] .
The d-dimers thus reflect the entire process of clot formation (coagulation) and its lyses [3] [8] .
Excess thrombin generation is detected by indirect markers (decrease in antithrombin, elevation of thrombin-antithrombin complexes, and increase in platelet turnover) and direct markers (mean) increase of 10% in thrombin generation potential. It can be detected and monitored in a delocalized way by the coagulation time and by an elevation of D dimers and a peak in plasmin generation [6] [7] [8] . Ducloy-Bouthors et al. (2010) demonstrated that fibrinolysis is early and intense [3] .
D-dimers can still be used in pregnant women as a biomarker of preeclampsia, if the average plasma concentration of D-dimers in the pregnant population has not yet been reassessed [20] .
Some studies recommend a gestational age-specific reference interval as the cornerstone of diagnosis of preeclampsia inflammation. However, preeclampsia inflammation is silent in its early stages of development and is sometimes a serious disease characterized by multisystem involvement during pregnancy [21] .
Although many studies have proven the involvement of preeclampsia as a cause of inflammation, in our circles no local study on D-dimers (markers) of this inflammation is available. Very few studies are conducted to determine the average concentrations of D-Dimers in our local populations and further studies will show how to set the cut-off point for D-Dimers based on the population average.
The aim of our study is to determine the D-dimer levels of preeclampsia pregnant women in our environment.
2. Material and Method
2.1. Method
A cross-sectional, multicenter, prospective study was conducted over a period of seven months in different prenatal consultation centers (PNC) in the city of Kinshasa. Sampling type was simple random.
The sample size of 119 was convenient. Healthy pregnant women from the second trimester attending PNC antenatal clinics were included in this study. The study group was 119 pregnant consulted between 2019 and 2022.
Inclusion criteria for the study were: women admitted in different PNC prenatal consultation centers between 24 and 40 weeks for a singleton pregnancy with symptoms of preeclampsia, without co-morbidities; and women admitted in different PNC prenatal consultation centers between 24 and 40 weeks for a singleton pregnancy without symptoms of preeclampsia and co-morbidities.
The sample was selected by consecutive non-probability sampling. After informed consent, Performa historical data was entered in isolation.
The eligible cases were preeclampsia pregnant women, consulted between 24 and 33 weeks for a single pregnancy with symptoms of preeclampsia, without co morbidities and non-preeclampsia pregnant women consulted between 24 and 40 weeks of gestation without any co morbidities.
During the first appointment, the pregnant women had obtained a request for a detailed blood test, the container needed to collect their urine and their imaging appointment to perform obstetric ultrasounds. It was agreed with each pregnant woman that we would contact her again upon receipt of all the laboratory results as well as the ultrasound and kidney results, in order to comment on the results concerning her and to define a possible prevention strategy.
All the pregnant women underwent a blood test and three obstetrical ultrasounds in each trimester of pregnancy.
2.2. Variables of Interest
Socio-demographic characteristics (Date of birth; Chronological age); Obstetricalgynaecologicalhistory (Parity and gesture).
2.3. D-Dimer Assay
Collection of blood samples and evaluation of D dimer.
A 3 ml venous blood sample to be collected from all pregnant women in a standard citrated tube correctly labeled (name, date and number). The sample was taken by peripheral venipuncture of the forearm using a Vacutainer system (Vacutainer, Becton-Dickinson, Oakville, ON, Canada), in a 5 ml tube containing 0.5 ml sodium citrate [22] .
Blood samples were centrifuged within 60 minutes of collection and stored at −80˚C until analysis. After sampling, the tubes in an upright position were left to stand for 60 minutes at room temperature. The samples were then centrifuged at 3000 revolutions (14 cm radius centrifuge) for 15 minutes to separate the plasma and the formed elements from the blood.
Assessment of D-dimer levels (µg/ml) was performed on a STAR automated coagulation analyzer, according to standard procedures; the concentrations of D-dimers will be measured by ELISA (gold standard of d dimer analysismethods). Serum d-dimer concentrations were determined in µg/ml. The reference values of D-dimers for the general population of these laboratory parameters of inflammation were (<500 µg/ml). Values below reference values were considered negative.
2.4. Ethical Considerations
The positive opinion of the obstetrics and gynaecology department was obtained.
2.5. The Opinion of the Ethics Committee
Recruitment was carried out anonymously after informed consent from each respondent.
The principle of respect for the privacy of the subjects of study as well as that of the confidentiality of the data collected. The data was kept in a bank with access reserved for the research team.
2.6. Statistical Analysis
All analyzes were performed using Statistical Package for the Social Sciences, software version 21 (SPSS Inc., Chicago, IL, USA).
Descriptive statistics were used to calculate the mean and standard deviation for continuous data (D-Dimer values during pregnancy). Gestate and parity: qualitative (ordinal) variables were expressed in terms of frequency and Continuous variables were expressed as mean ± standard deviation or median (interquartile range), depending on their distribution.
Categorical variables were reported as absolute numbers and percentages. The effect of gestational age on D-Dimer levels was tested by univariate linear regression analysis.
The sensitivity and specificity of the D dimer assay was tested using the Roc curve.
Differences between the two groups for qualitative and quantitative variables were analyzed by Student test, U Man-Whitney test, Kruskal Wallis test respectively and analysis of variance with Spearman’s post hoc. The relationship between plasma D-dimer levels and between preeclampsia and no-preeclampsia pregnant women by gestational age was determined using the U Man-Whitney post hoc test. Statistical significance was set for p < 0.05.
2.7. Operational Definitions
Operational terms have been defined as follows:
Age at birth as an age calculated from date of birth and last birthday
1) Weight in kg and height in cm.
2) Parity as the number of previous deliveries ordinal qualitative variables.
3) Gestation as the number of previous pregnancies.
4) Gestational age defined as a pregnancy age calculated according to the first trimester ultrasound protocol.
5) Preeclampsia as de novo gravid blood pressure (systolic/diastolic ≥ 140/90 mm hg occurring after the 20th gestational week.
6) D = Dimers as biological markers, at the ordinal scale.
・ Have lived in the Mabulu 2 quarter in the Commune of Selembao for at least two years,
・ have a child under five who has had a recent episode of fever,
・ Being able to speak French or Lingala,
・ Accept freely and in an informed manner to participate in the studyWhitney.
3. Results
Of all the subjects studied, the data analysis focused respectively on 35/119 (i.e. 31.1%) preeclampsia pregnant women and 84/119 (i.e. 68.9%) no-preeclampsia pregnant women. The mean age of 119 pregnant women consulted in PNC was 38.36 ± 4.2 years (interval to 16 - 49 years) (Table 1).
In a control group, 84 pregnant were physiologically normal pregnancies. Preeclampsia was diagnosed in patients with high blood pressure and qualitative proteinuria in urine collection. Between two groups, the age (p < 0.001), the parity (p ≤ 0.001), the BMI and the gestational age were significantly different.
Between preeclampsia and no-preeclampsia pregnant women, the gestational age and the BMI were significantly different (p-value < 0.001 and 0.003) (Table 2).
The rock curveshows that, the test used to measure the d-dimer was a better test (Graph 1).

Graph 1. The rock curve for sensitivity and specificity of d-dimer assay test.
![]()
Table 1. General characteristics of the study population.
![]()
Table 2. Maternal variables and pregnancy evolution.
**Significant difference at the 0.01 level; ***Significant difference at the 0.001 level.
The findings of our study show that during all pregnancy normal or preeclampsia, the D-dimer levels increase under that conventional D-dimer of 500 µg/ml (Graph 2).
Depending on the age of pregnancy (Graph 3), the concentrations of D-dimers were significantly different and a statistically significant correlation was observed between the two variables (r’ = −0.311; p-value < 0.001).
Table 3 represents the serum concentrations of D dimers in normal and preeclampsia pregnancy. Between the two groups, the serum concentrations of D dimers were significantly different (p-value < 0.001). They were very high (2723 µg/ml) for preeclampsia pregnant women compared to those of no-preeclampsia pregnant women (639 µg/ml).
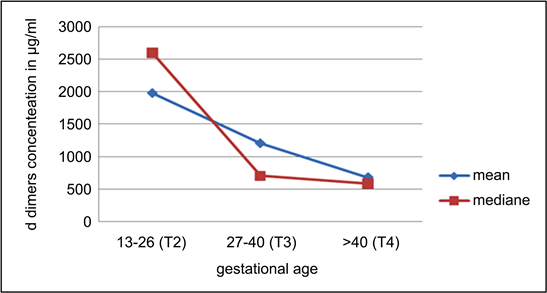
Graph 2. Concentrations of D dimers by gestational age.
![]()
Table 3. Serum concentrations of D dimers in normal and preeclampsia pregnancy.

Graph 3. D Dimer during pregnancy.
4. Discussion
The findings of our study show that during all pregnancy normal or preeclampsia, the D-dimer levels increase under that conventional D-dimer of 500 µg/ml (Graph 2). This is because even normal pregnancy is associated with physiological increase in D-dimer levels. So new thresholds of D-dimer should be defined before utilizing this test for ruling out intravascular coagulation disseminate in pregnancy [23] .
During pregnancies complicated by preeclampsia or progressing normally, the D-dimer values are above the conventional threshold of 500 µg/ml. Contrary to several authors (Hedengran et al. 2016 [17] , Murphy et al. 2016 [16] ), we measured serum D-dimer levels only during the last two trimesters of pregnancy (Table 3). This fact bears witness to the general behavior of pregnant women in our environment. Indeed, there is no binding law for declaration of pregnancy at its beginning, pregnant women consulting in the first trimester only in the crisis situation.
However, our study also shows that D-dimer levels increased significantly during the later trimesters of pregnancy (Graph 3). If in the studies of Hedengran et al. (2016), the increase in D-dimer is maximal in the third trimester, in our study, the levels of D-dimer regress at this age of pregnancy [17] .
The work of Bao et al. (2017) on D-dimer assays in preeclampsia women has shown a strong correlation between D-dimer assays and the diagnosis of preeclampsia [21] .
Like Goldhaber et al. (2012), the sensitivity of D-dimer assays was maximal as a function of the duration of preeclampsia [9] . In our work, the sensitivity and specificity of D-dimers were close to 100% in the third trimester of pregnancy (Graph 2). With preeclampsia have shown a strong correlation between D-dimer assays and the diagnosis of preeclampsia.
D-dimer values, in a properly risk-stratified population, can be a diagnostic test for disseminated intravascular coagulation. However, in our study the D-dimer values algorithm was not established.
Goldhaber et al. (2012) reported that when serum D-dimer values were elevated at 500 µg/mL, sensitivity of the dosage was 0.90% in the diagnosis of preeclampsia [9] .
In the literature (Bao et al. (2017) higher values of D-dimers are often associated with high feto maternal mortality rates [21] . However, our study being limited could not confirm this correlation.
The following reference ranges have been defined: first trimester: 169 - 1202 g/L, second trimester: 393 - 3258 μg/L and third trimester: 551 - 3333 μg/L. Hedengran et al. (2016) suggests a gradual increase in D-dimers throughout pregnancy. Murphy et al. (2016) found that pregnancy increases the concentration of D-Dimer progressively from conception to birth at all gestational ages [17] . In our study, on the contrary, the ranges of concentrations of D-dimer (Graph 3) decrease from the second trimester to the end of pregnancy.
A study by Kovac et al. (2010) showed a linear relationship between pregnancy and D-dimer concentration. It concluded that 84% of women had a normal D-dimer in the first trimester, 33% had a normal concentration in the second trimester, and 1% had a normal concentration in the third trimester if the threshold of 230 µg/mL (0.23 μg/ml) was used [10] . They found that the level of D-Dimer increased with the progression of pregnancy from the first trimester to the third and reached the highest level during the latter noted that D-Dimer was 6.7 - 7.6 times higher in the first trimester, while 2.0 - 3.8 times higher in the 3rd trimester when associated with thrombosis and outcomes positive ultrasound (p < 0.001).
For the third trimester, we noted a D-Dimer value > 5 times compared to the normal threshold (the maximum value being 5 µg/ml) when it is not even associated with preeclampsia.
The lack of sponsors is the main reason, as both the research team and the pregnant women do not have an ad hoc budget Notwithstanding these limitations, ours study established for the first time in our circles that disseminated intravascular coagulation is a real risk during both normal and complicated preeclampsia pregnancy.
Consistent with previous studies, this study also suggests that if a threshold for D-dimers in pregnancy is warranted, then it should be specific to gestational age and should be interpreted with great caution [24] [25] .
Pregnancy has a strong association with disseminated intravascular coagulation, and at the same time D-dimer levels are also physiologically elevated during pregnancy. Although the use of D-Dimeras, an indicator of disseminated intravascular coagulation, is highly beneficial, especially in resource constrained settings due to its rapid and non-invasive characteristics, many researchers have reached a consensus according to which the cut-off level should be high to screen only true positives. This study has many advantages, including a reasonable sample of pregnant women observed for levels of D-Dimers.
5. This Study Has Several Limitations
First of all the major weakness of the previously published randomized, controlled study is its open-label, unblinded design. A second limitation is the limited number of patients in the two groups. Finally the third, we did not calculate the Algorithm of thrombotic risk like several authors (Ducloy-Bouthors et al. 2016 [3] ; Mallaiah et al. 2015 [26] ; Collins et al. 2014 [8] ; Olson et al. 2013 [27] ; Su et al. 2012 [7] ; Cortet et al. 2012 [28] ; Hui et al. 2012 [19] ).
The lack of sponsors is the main reason for this, the team of researchers as well as the pregnant women having no ad hoc budget.
Notwithstanding these limitations, our study has established for the first time in our circles that disseminated intravascular coagulation is a real risk during pregnancy, both normal and complicated with preeclampsia. We recommend particular attention during childbirth, in particular to avoid the use of instruments such as the suction cup or to take samples from the scalp of the newborn.
6. Conclusion
D-Dimers are elevated in patients with Preeclampsia and provide valuable diagnostic and prognostic information. In pregnant women with Preeclampsia and elevated D-Dimer, a diagnosis intravascular coagulation should be considered. D-Dimer might be a useful complementary tool to the current diagnostic work-up of patients with suspected intravascular coagulation.
Author Contributions
Kamba, Tozin, Kitenge, Badibanga, contributed to the design of the study; Badibanga, Kamba contributed to data collection; Kamba, Kakwaka, Atuba contributed to data analysis; Kamba, Kakwaka, Atuba contributed to the interpretation of the collected data; and Kamba wrote the first draft of the manuscript, and all the authors contributed to its final changes. They readied and accept final namuscript. Atuba contributed to submitting the manuscript to the journal.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of the Public health school of Kinshasa. Written informed consent was obtained from each subject.