This study is concerned with describing the thermodynamic equilibrium of the saturated fluid with and without a free surface area
A. Discussion of the role of
A as system variable of the interface phase and an estimate of the ratio of the respective free energies of systems with and without
A show that the system variables
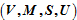
given by Gibbs suffice to describe the volumetric properties of the fluid. The well-known Gibbsian expressions for the internal energies of the two-phase fluid, namely
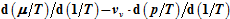
for the vapor
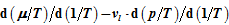
and
for the condensate (liquid or solid), only differ with respect to the phase-specific volumes
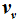
and
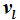
. The saturation temperature
T, vapor presssure
p, and chemical potential
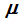
are intensive parameters, each of which has the same value everywhere within the fluid, and hence are phase-independent quantities. If one succeeds in representing
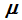
as a function of
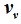
and
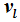
, then the internal energies can also be described by expressions that only differ from one another with respect to their dependence on
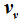
and
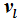
. Here it is shown that
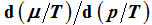
can be uniquely expressed by the volume function
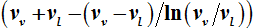
. Therefore, the internal energies can be represented explicitly as functions of the vapor pressure and volumes of the saturated vapor and condensate and are absolutely determined. The hitherto existing problem of applied thermodynamics, calculating the internal energy from the measurable quantities
T,
p,
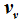
, and
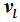
, is thus solved. The same method applies to the calculation of the entropy, chemical potential, and heat capacity.