1. Introduction
Lithium ion secondary batteries have been noticed for many applications to high performances recently, and the developments have been performed [1] . For example, material development of negative electrode has been concerned with safety. The material is currently graphite, which has layer structure and is intercalated by lithium ions into the layers. On the other hand, the graphite is not safe to expand with overcharge to intercalate amount of lithium ions over the stoichiometry into the layer. Therefore, noble materials have been investigated instead of the graphite to prevent ignition by internal short-circuit [2] . The noble materials need some properties, whose crystal structure shows no change and high stability with intercalate lithium ions. Furthermore, the synthesis is easy and inexpensive to mass production. Here, we have studied Li4Ti5O12 as superior negative electrode materials [3] .
Li4Ti5O12 has spinel structure and LiMn2O4 does likewise, which is applied to the positive electrode in the lithium ion secondary battery. The properties of Li4Ti5O12 are hardly expanded by the overcharge, and high stability in cycles of discharge and charge. The synthesis methods are known as solid phase synthesis, sol-gel process, hydrothermal synthesis, coprecipitation method and vapor phase deposition method. Especially, the solid phase synthesis is a good method for industrial processes, which is simple and easy to control composition of a chemical compound. However, it has some problems, such as a low reactivity, a control of fine particle size and a production of by-products. The synthesis developments have been performed to solve them [4] [5] .
Consequently, the synthesis method is needed to produce Li4Ti5O12 of a single phase, fine nano-size particles and a high crystalline in order to show high performance as the battery. A synthesis process has been performed by sintering with low temperatures by a two-step process of a pre-sintering at 400˚C - 500˚C and a sintering at 700˚C - 750˚C, because we have tried to synthesize a single phase and fine nano-size Li4Ti5O12.
2. Experimental
Li4Ti5O12 synthesis was carried out by using CH3COOLi・2H2O (Wako Pure Chemical Industries, Ltd.) as a Li source that was melted with low temperature at about 300˚C, and anataseTiO2 (Toho Titanium Co., Ltd.) as a Ti source. The first synthesis process was mixed with the CH3COOLi・2H2O and TiO2 with Li:Ti = 4:5 by ball milling (Fritsch, Pulversitte 7) at 1 h, rotating speed at 320 rpm and orbital speed at 110 rpm in agate mortar and balls. The mixed powder was pre-sintering at 400˚C, 450˚C and 500˚C with 10˚C/min, 1 h in air, and a precursor was formed. Furthermore, the precursor was mixed by the ball milling at 1 h similarly, and was sintered at 700˚C and 750˚C with 10˚C/min at 1 h in air.
The obtained powder was identified by XRD (Rigaku Corp., Rint 2000) at scanning step 0.02 deg and scanning speed 5 deg/min by CuKα, and was also measured by BET specific surface area (Shimadzu Corp., FlowSorb III 2305) at 0.1 g sample, gas flow rate 80 cm3/min in N2 and current 50 mA, after degassing the sample with heating at 160˚C at 2 h. Particle size of the obtained sample was observed by FE-SEM (Hitachi, Ltd., S- 4200), and crystalline estimation tried with TEM (JEOL Ltd., JEM-2100).
3. Results and Discussion
3.1. Pre-Sintering
Precursor was formed by pre-sintering the CH3COOLi・2H2O and TiO2 mixed powder at 400˚C, 450˚C and 500˚C in air, and showed in Figure 1. These indicated unreacted TiO2 and sub-phase Li2TiO3 of Li4Ti5O12 under any temperature. This relationship is known as a phase diagram of Li2O-TiO2 among TiO2, Li2TiO3 and Li4Ti5O12, which shows an accurate mixed rate at Li and Ti atoms [6] . Ti site of Li2TiO3 and Li4Ti5O12 is common in center of an octahedron, while Li site exist a tetrahedron in the case of Li2TiO3. Hence, Li2TiO3 shows inactivity as the electrode not to charge and discharge Li ions [7] .
SEM images were shown about the precursors in Figure 2. These indicated that average grain size increased with the pre-sintering temperature at 50 nm, 70 nm and 75 nm under 400˚C, 450˚C and 500˚C. Simultaneously,
![]()
Figure 1. XRD patterns of the samples pre-sintered at 400˚C (black line), 450˚C (blue line) and 500˚C (red line), which show ▲; Li2TiO3 and ×; TiO2.
![]()
![]()
![]()
Figure 2. SEM images of the samples for the pre-sintering temperature of (a) 400˚C, (b) 450˚C and (c) 500˚C.
BET specific surface area presented 27.7 m2∙g−1, 26.6 m2∙g−1 and 26.2 m2∙g−1 under 400˚C, 450˚C and 500˚C. The images of the grain observed two types as deference of contrast at 400˚C in Figure 2(a), and showed a uniform contrast with the high temperature. These results might indicate a localization of unreacted TiO2 and sub- phase Li2TiO3 in keeping with XRD patterns. This tendency may confirm TEM images in Figure 3, which will show a low crystallization with corresponded to XRD not to observe facet areas.
3.2. Sintering
XRD peaks of samples ware showed at sintering temperature of 700˚C and 750˚C in Figure 4 and Figure 5. Peaks intensity of Li2TiO3 decreased, while those of Li4Ti5O12 increased with pre-sintering temperature at 700˚C sintering in Figure 4. Single phase of Li4Ti5O12 was obtained at the pre-sintering temperature of 500˚C in Figure 5, however the peaks intensity indicated the similar tendency about pre-sintering temperature with the sintering temperature of 700˚C. Here, we defined a single phase rate to estimate it, which utilized the peaks area of them at shown in following equation.
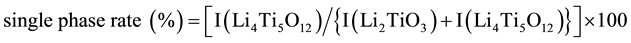
The value at 700˚C was 80%, 84% and 86% at the pre-sintering temperature of 400˚C, 450˚C and 500˚C, which were advantageous with the high pre-sintering temperature. Furthermore, the value at 750˚C was 92%, 99% and 100% similarly, and we could obtain the single phase Li4Ti5O12 at 750˚C. These results will show that the sub-phase Li2TiO3 give effects for the Li4Ti5O12 synthesis.
A surface separation of (002) face of Li2TiO3 at 4.80 Å is very close to that of (111) face of Li4Ti5O12 at 4.83 Å in the spinel structure, which Li4Ti5O12 would be able to form from Li2TiO3 with holding the structure [8] . The knowledge will show that Li4Ti5O12 was synthesized via Li2TiO3 by the pre-sintering, and the single phase was obtained at 750˚C in this process.
SEM images of the samples showed in Figure 6. The average grain size was observed at about 90 nm in any samples at sintering temperature of 700˚C in Figures 6(a)-(c), and the BET specific surface area was about 10.5 m2∙g−1 for any samples. Similarly, it was at 110 nm under 750˚C in Figures 6(d)-(f), and was 7.3 m2∙g−1, 6.3 m2∙g−1 and 6.1 m2∙g−1 in the specific surface area. The grain size of the single phase Li4Ti5O12 have reported about 600 nm in the solid phase synthesis under 850˚C at 12 h by boll-milling [9] - [11] , and Guerfi has especially reported the grain size of 150 nm by mixing graphite into the ball mill [9] . On the other hand, we could synthesize that of grain size of 110 nm via Li2TiO3 at 750˚C by the two steps sintering method.
TEM images of the samples showed in Figure 7, and the shape of the grain indicated uniformity in any samples. Furthermore, morphology was improved at the sintering temperature of 750˚C compared with at 700˚C, and facets in the grain were observed by a crystal growth. The morphology have been reported about the synthesis by using nano-particle or nano-wire TiO2, which have described that the nanoparticle or nanowire Li4Ti5O12 was obtained on keeping the TiO2 morphology like a mold [12] [13] . This knowledge will provide a synthesis mechanism for this two steps sintering method, which the (002) face of Li2TiO3 plays a role of the mold in the spinel structure. These may show the reason why the surface separation of the (002) Li2TiO3 has very close it in the (111) Li4Ti5O12.
![]()
![]()
![]()
Figure 3.TEM images of the samples for the pre-sintering temperature of (a) 400˚C, (b) 450˚C and (c) 500˚C.
![]()
Figure 4. XRD patterns of the samples sintered at 700˚C with the pre-sintered temperature of 400˚C (black line), 450˚C (blue line) and 500˚C (red line), which show ●; Li4Ti5O12 and ▲; Li2TiO3.
![]()
Figure 5. XRD patterns of the samples sintered at 750˚C with the pre-sintered temperature of 400˚C (black line), 450˚C (blue line) and 500˚C (red line), which show ●; Li4Ti5O12 and ▲; Li2TiO3.
4. Conclusion
Single phase Li4Ti5O12 was synthesized by the two-step sintering via Li2TiO3. The method especially obtained the fine nanoparticles at about 110 nm and 6.1 m2∙g−1 under sintering temperature of 750˚C. The process will bring about expectation for the mass production in the industry.