Study on the Mechanism of Heterogeneous Catalysis (1) —The Measurement of Equilibrium Pressure of Reaction BaCO3 + C = BaO + 2CO, the Chemical States of Fe in Reaction Tank, and Chemical Reaction Model Catalytic Cycle Mechanism (CRMM) ()
1. Introduction
The pack carburizing of steel part surface is an ancient method. The applied history in industrial production has more than one hundred years. In order to accelerate carburizing rate, improve the quality of carburized layer, the accelerator (or catalyst) is always introduced into carburizing agent. The most commonly used accelerator is the BaCO3. The BaCO3 can speed up the carburizing process, it is no any doubts. However, about the catalytic mechanism, it is said to be opinion vary, independent, and it has not yet been final conclusion. It is a worry that in the catalysis academia, some scholars have called incredibly the CRMM as a “principle”. It is really disturbing.
It is well known that the catalyst to speed up the carburizing process is due to speed up carbon gasification reaction rate and increased CO concentration. However, as for what was the mechanism for increasing the CO concentration, each has his own words. The carbon gasification reaction which also is called as Boudouard reaction is a very important industrial reaction. The gas production, iron making and smelting copper in blast furnace, sponge iron production in tunnel kiln etc. rely entirely on this reaction. Therefore, there are many studies on catalysis mechanism of carbon gasification reaction.
The most commonly adopted is the “Oxygen Transfer Mechanism or Theory- OTM or OTT”. In fact, the OTT is also CRMM.
The main points of the OTT are oxidation-reduction cycles. In 1981, D.W. Mckee [1] , referred to 152 literatures, Wrote an article for 152 pages, and reported the research results on the catalytic effects of Alkali-Metal oxides and salts, Alkaline Earth oxides and salts, Transition metals and oxides, Noble Metals and miscellaneous catalysts on the C + O2, C + CO2, C + H2, C + H2O four reaction with CAEM (Controlled Atmosphere Electron Microscopy) a new technique. In conclusion, he said “Although it is not yet possible to explain all the observed catalytic effects within one all-encompassing, mechanistic framework, on balance specific oxidation-reduction cycles have been conspicuously successful in interpreting the effects of alkali metal salts, transition metals and oxides, and the noble metals in the various types of carbon gasification reactions. However, many details of the complex catalytic phenomena still remain obscure and await elucidation”. Obviously, he recognizes CRMM.
In 2009, Deutschmann et al. [2] , referred to 628 literatures, they worked together to publish a book
. They, in the author’s opinion, comprehensively summarize the heterogeneous catalysis research and production results more than 100 years so far and many. But, in this book they have repeatedly called the CRMM as a “principle”. One is Sabatier’s principle published at 1902, and another is Boudart’s “principle” published at 1992. Author called it as a “S-B principle”. The original paper wrote: “The Sabatier’s ‘principle’ proposes the existence of an unstable intermediate compound formed between the catalyst surface and at least one of the reactants. This intermediate must be stable enough to be formed in sufficient quantities and labile enough to decompose to yield the final product or products”. The Boudart’s “principle” is called the most fundamental principle in catalysis. “The most fundamental principle is that of the catalytic cycle, which may be based on redefinition of a catalyst by Boudart: “A catalyst is a substance that transforms reactants into products, through an uninterrupted and repeated cycle of elementary steps in which the catalyst is changed through a sequence of reactive intermediates, until the last step in the cycle regenerates the catalyst in its original form”; “The activity of the catalyst is defined by the number of cycles per unit time or turnovers or turnover frequency (TOF; unit:S
−1). The life of the catalyst is defined by the number of cycles before it dies.”
According to this “S-B principles”; it can be summed up three conclusions:
1) The catalysts had to participate in chemical reactions.
2) In reacting processes had to generate intermediate compounds. The intermediate was stable and had a certain number and easy decompose.
3) After a series of reactions, and eventually regenerated the catalyst in its original form.
There are many kinds of catalyst. If according to the CRMM, there must be a lot of catalytic cycle reactions and intermediates. Therefore, all kinds of catalytic cycle mechanism of chemical reaction model all called CRMM by author. The CRMM is widely popular, and is deep roots in academia.
In the literature, the oldest explain in regard to the catalytic activity of barium carbonate was the carbon dioxide emissive theory. This theory considered that the Barium carbonate due to thermal decomposing gives off carbon dioxide, the reaction of carbon dioxide with carbon gives birth to carbon monoxide, increased the carbon monoxide concentration, thus accelerating the rate of carburizing. It turned out that CaCO3, MgCO3 to emit a large amount of carbon dioxide is inert, but the BaCO3, SrCO3 not emitted carbon dioxide has good catalytic activity, so the carbon dioxide emissive theory was give up, and then the theory of carbon monoxide generating is adopted. CRMM at present is also widely popular, it is as follow:
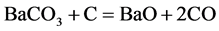 (1)
(1)
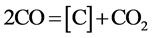 (2)
(2)
 (3)
(3)
 (4)
(4)
It can be see that the BaCO3 is repeated decomposition and generation. The reaction (2) occurred on the surface of steel part is carburizing reaction. In practice, the thermodynamics data calculation can completely prove that the cyclic reaction does not occur in the carburizing box condition. In order to make reader convinced, the equilibrium pressure measurement of BaCO3 + C = BaO + 2CO reaction have been done. Look at this reaction at carburizing temperature in carburizing box condition is able to or cannot to occur. At the same time, based on years of experience on producing reduce iron powder, look at the oxidation-reduction cycle reactions of iron can or cannot occur in reduction reaction tank condition.
2. Experiment
BaCO3 + C = BaO + 2CO reaction equilibrium pressure measurement is carried out at a vacuum system. The vacuum system is equipped with oil pressure gauge, mercury manometer, McIntosh vacuum gauge, vacuum pump and diffusion pump etc.. Before the measurement, the vacuum system included sample chamber and reactant was evacuated by vacuum pump for vacuum degassing for three days. Until after the vacuum system is indeed sealed, there is no leakage phenomenon for a long time, and then began to do experimental measurement.
Reactant or samples consists of 8 grams BaCO3 (CP grade) and 1.2 grams graphite powder (ashes; 0.4%) to get mixed to uniform, and then the reactant is charged into quartz tube. The tube length of diameter Φ12XΦ15 is 500 mm, one end of tube is sealed, and another end is joined to vacuum system. The length of reactant layer in tube about is 75 mm. One end of the loading tube is heated in a vertical tubular furnace with an inner diameter of 35 mm and a length of 300 mm.
On the process in heating up, the equilibrium pressure of reaction was measured step by step. The stay time is respectively 40 - 420 minutes at reaction equilibrium.
From oil pressure meter instructions, the fluctuation of equilibrium pressure value caused by temperature fluctuations, at every equilibrium point, is lower than 0.2 mm Hg column.
X-ray analysis [3] shown that alkali type barium carbonate (BaO-BaCO3) does not exist. But, there are BaO and BaCO3 eutectic compounds between 1070˚C - 1150˚C. Therefore, in this temperature range 756˚C - 976˚C, this reaction of barium carbonate with carbon should be occurred in accordance to the reaction (1).
The Pressure measured by pressure gauge should be the reaction equilibrium pressure. Of course, this gas phase contains a amount of carbon dioxide. The CO2/CO2+CO% ratio values can be obtained from reaction (4) equilibrium constant. However, when the temperature is higher than 900˚C, the pressure is almost entirely from carbon monoxide. Anyway, it does not affect the result discussion.
The reaction equilibrium pressure measured of experiment is given in the Table 1.
![]()
Table 1. Reaction equilibrium pressure of BaCO3 + C = BaO + 2CO.
3. Results and Discussion
1) BaCO3 catalyst
According to the basic principles of thermodynamics, the thermodynamics data of the carbon gasification reaction were combined with the equilibrium pressure data obtained of this experiment, We have obtained the equation of the relationship between Barium carbonate decomposition pressure with temperature. It is as follow:

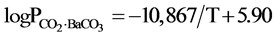
In Table 2, the results of the direct determination by Lander [4] with static method are also listed. To compare the results, the experiment results are higher than the static method results, especially at the low temperatures. The reason is interfered by the carbon dioxide. However, when the temperature is higher than 900˚C, the interference can be neglected. Therefore, it is said that the result of the experiment was completely reliable.
Will the cyclic reaction (1) be take place in carburizing box?
In order to judge whether the reaction (1) can occur in the carburizing box condition, it is necessary to know the gaseous composition in the carburizing box condition, i.e. the environmental conditions.
Pack carburizing box is a closed system. The box is filled by coke carbon powder, steel parts, air, and small amounts of BaCO3 catalyst in the beginning. On the process to heat up, a lot of air is expelled from the box, and on the other hand, the reaction of carbon with remnant oxygen in box takes place, as follow:
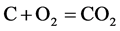 (5)
(5)
 (6)
(6)
According to the (5), (6) reaction equilibrium constant (1200˚K, logKp(5) = 17.35, logKp(6) = 19.25), we can seen that the reaction (5) and (6) was come out almost to the end at carburizing temperature, the oxygen content in box was extremely small. Therefore, the reaction (5), (6) can be neglected, there are only (2), (4) two reactions in carburizing box.
Reaction (4) occurs on the surface of carbon, and the reaction (2) takes place on the surface of steel parts. The active carbon produced by reaction (2) was ab-
![]()
Table 2. Decomposition pressure of BaCO3.
sorbed by iron for carburizing. Therefore, the gaseous composition in the carburizing box completely depends on the equilibrium reaction (4) and (2). After the carbon content of iron at parts surface had once reached saturation, the gaseous composition in the carburizing box completely determines the reaction (4). According to the thermochemistry data, it is easy to calculate the reaction equilibrium gaseous composition.
It can be seen from Figure 1 [5] that the CO concentration in the equilibrium gas phase increased rapidly with the increasing of the carbon content in the γ-Fe, and then it is saturated by carbon. At 915˚C, the equilibrium gaseous composition corresponding to 1.2% C of γ-Fe should fall at point “b”, and the equilibrium gaseous composition of carbon gasification reaction should fall at “a”. It can be seen that the two points were very close. According to the results of Mcquid’s [6] analysis on the gaseous composition in carburizing box, it is considered that the gaseous composition in the carburizing box was almost all CO and CO2. Therefore, it is more reasonable to think that the sum of CO and CO2 is equal to one atmosphere. That is, pCO + pCO2 = 1atm.
As a result, the data listed in Table 3 can be used as basis to discuss. This should be no doubt about it.
Can the BaCO3 + C = BaO + 2CO cyclic reaction be occur in the carburizing box condition?
The CRMM thinks that the barium carbonate catalyst is repeatedly decom-
![]()
Figure 1. Fe-CO-CO2 equilibrium diagram.
![]()
Table 3. Equilibrium gaseous composition of reaction (CO2 + C = 2CO).
![]()
Figure 2. Equilibrium pressure of BaCO3 + BaO + 2CO reaction.
posed and generated. The intermediate compound is BaO. Some scholars [7] even think that the metal barium is an intermediate, and so on. It is completely ignoring the basic principles of thermodynamics. The most basic requirement on repeatedly generating and decomposing cycle of Barium carbonate is that the pressure between equilibrium pressure p1 of reaction (1) and pressure p2 of CO in carburizing box, namely the CO pressure in the environment, must be equal. That is p1 = p2,.
From Figure 2, two lines intersecting points can to meet the P1 = P2 (P1 line extension). The temperature of intersection point is about 1040˚C. That is to say, only at 1040˚C, the repeatedly decomposition and generation cyclic reaction will occur, once it leaved this temperature or the environment pressure changed, the equilibrium reaction will immediately damage, decomposition-generation cyclic reaction will immediately disappeared. At the carburizing temperature (940˚C), P2 is much higher than P1, Hence, Barium carbonate can only exist in the form of BaCO3, barium oxide can't appear, the decomposition-generation cyclic reaction is never to take place. If, in accordance with the CRMM, the Barium carbonate should be inactive, but it is actually a good catalyst.
The CRMM not only unable to illustrate that the different catalysts have catalytic activity at the same temperature, and can not account for that the same catalyst has catalytic activity over a wide temperature. Because of the decomposing-generating or oxidizing-reducing repeatedly are unlikely to occur on the different catalysts at the same temperature, and also are unlikely to occur on the same catalyst over a wide temperature range. Therefore, it is said that the CRMM is not simply credible. TO call it as a “principle” is clearly more inappropriate.
2) Iron catalyst
Can the oxidation-reduction cycle reactions of iron be occur in reduction reaction tank condition?
Sponge iron producing and pack carburizing are very similar. The reduction tank is filled with iron oxide powder and coke powder, and the carburizing box is filled with coke powder and steel parts. The environmental conditions are completely identical.
The author was engaged in the iron powder production for 18 years. The composition and flow rate of gas omitted from reduction tank at a 38.5 meters long tunnel kiln have been measured for several times. The catalytic and poisoning effects of Fe, Co, Ni, Cu, Ag, SiO2 on the reducing process have been studied in order to verify ECDAT or ODRM (Electron Cyclic Donate-Accept Catalysis Theory-ECDAT or Electron Orbit Deformation-Reversion Cycle Catalysis Mechanism ?EODRCM or ODRM).
For the metallurgist, Figure 3 is a significant curve.
From Figure 3 [8] , according to the CO2/CO2 + CO% of reduction gas composition changes, we get three conclusions:
1. The reducing process is step by step, that is: Fe2O3-Fe3O4-FeO-Fe. The time of reducing process of FeO + CO = Fe + CO2 is the longest, nearly about 28 hrs.
2. To compare the reduction gaseous composition CO2/CO2 + CO% with the equilibrium gaseous composition of FeO + CO = Fe + CO2 and the CO2 + C = 2CO reaction, the CO/CO+CO% ratios are close to ratios of reduction reaction equilibrium, and away from the ratios of carbon gasification reaction equilibrium. Thereby, we can conclude that the carbon gasification reaction is the rate-controlling step.
3. There are a “reduction end point” between reducing process and carburizing process. The reducing process and carburizing process are sharply separated. Until the end of reducing process, the carburizing began.
According to the operating instructions, in order to produce high quality iron powder, the sponge iron ingot quality must be checked every day, whether the production process is reasonable. Years of practice has proved that as long as there are a small amount of black iron oxide on the sponge iron ingot section, the carbon content in sponge iron is always less than 0.03%, it is shown repeat-
![]()
Figure 3. Composition and flow rate of gas emitted from reaction tank in 38.5 meters tunnel kiln.
![]()
Figure 4. Catalytic activity of iron in the reduction process with coke.
![]()
Figure 5. Section of reduction reaction tank.
edly that the reducing process was still in the reducing stage, and not yet entered into carburizing stage. The chemical state of iron as a catalyst in the coke powder layer can only appears in the form of metallic iron, iron is stable phase, iron oxide is unstable phase. The oxidation-reduction cyclic reaction of Fe can't occur in the coke powder layer.
Figure 4 [9] shown the results of the experiment on the catalytic activity of Fe in the reducing iron oxide by carbon. According to the three indicators, namely; flow rate and composition of gas emitted from reaction box, and the carbon content in sponge iron obtained after reduction, the carbon content in sponge iron is 0.59% at the presence of 8%Fe catalyst and 0.35% at the absence of catalyst, it shown completely that the iron has good catalytic activity.
Figure 5 shown a cyclic reaction in the reduction tank. The carbon gasification reaction takes place in the carbon powder layer, it has generated the carbon monoxide to diffuse to iron oxide powder layer, and then it with iron oxides take place the reduction reaction, the carbon dioxide produced by reduction reaction to diffuse to coke powder layer, so the cycle continues, Until the iron oxide have been reduced completely to metallic iron. When the carbon content of iron reaches saturation, the activity of carbon is equal to 1, the cyclic reactions have finished.. Now that a large amount of iron oxide can put to reduce into metallic iron, the iron in the coke powder layer can only appears in the form of metallic iron in reducing stage, and can only appears in the form of Fe3C after carburizing, so that oxidation-reduction cycle reaction of iron absolutely can not occur in the coke powder layer, this is a very simple reason. It is completely consistent with the basic principles of thermodynamics. Some scholars even said that the basic principle of thermodynamics does not provide a clear direction on the catalysis. If you left a basic principle to discuss the problem, it's not messed up, re-
![]()
Figure 6. Stable area of Fe, FeO, Fe3O4 at carbon present.
ally incredible.
Figure 6 is a figure from metallurgical textbooks [10] . From this figure, it can seen also that the stable phase was metallic iron at the temperature higher than about 708˚C (T2) in the carbon present case. Between T1-T2 (about 655˚C - 708˚C), the stable phase is FeO. Under 655˚C, the stable phase is Fe3O4. It clearly tell that the oxidation-reduction cyclic reactions are absolutely unlikely to occur at the reducing temperature (1000˚C).
Said so many, it is only in order to explain that the oxidation-reduction cyclic reactions can’t occur in the reduction reaction tank condition. For metallurgists, it is redundant, due to these are the most common basic knowledge, they will never to accept the oxidation-reduction cyclic reaction or CRMM to account for the catalytic mechanism.
3) Noble metal catalyst
It may be noted in passing that the noble metals are an active catalyst for carbon gasification reaction. However, it is even more incredible that some scholars actually think that the noble metal catalysts also can undergoes an oxidation- reduction cycle reaction.
In nature, the noble metals can appear in the free metallic state, because of the decomposition pressure of its oxide is much greater than the partial pressure (0.21 atm.) of oxygen in the air, so it can only appears in metallic state form. The iron can not appears in the metallic state, because of the decomposition pressure of iron oxide is much lower than the partial pressure of oxygen in the air, the Fe can only exist in Fe2O3 forms. The lime can agglomerate, because of the decomposition pressure of CaCO3 is much lower than partial pressure of carbon dioxide in the atmosphere. These are the most basic knowledge. In carburizing boxes or reduction reaction tank, if it is considered that the noble metals will also occur oxidation-reduction reaction cycle, that is really Arabian nights.
Materials of a very wide range have been found to be active catalysts for the carbon gasification reaction. If, in accordance with CRMM, there will be a lot of chemical reactions and many intermediate compounds, everyone has everyone’s argument, let us dazzling. The inevitable chaos situation will appear, let us not know what to do.
We can also cite many examples to account for that the CRMM is not credible. The space is Limited, not much more.
4. Conclusions
1) Experiment results and theoretical analysis have demonstrated that the BaCO3, Fe, noble metal catalyst cannot repeatedly decompose and generate, oxidize and reduce in the carburizing box or reduce tank condition. Catalysts do not participate in chemical reactions, they are stable phase. So the CRMM which is widely popular for a long time does not accord with the basic principles of thermodynamics. It is not credible. Therefore, it is inappropriate to call the CRMM as a “principle”.
2) Because of the catalytic cycle reactions do not at all exist. So that to measure catalyst activity size and life by TOF is apparently out of thin air.
Because of the lack of the correct basic theory act as a guide in scientific research and production field, it lost its way.
3) It seems that the ECDAM (Electron Cyclic Donate-Accept catalysis Mechanism) or EODRM (Electron Orbital Deformation-Recovery Cycle catalysis Mechanism) proposed by author can satisfactorily account for many experimental results, including catalysis and poisoning, and the arrangement of activity size of various catalysts. It has not yet found with a big contradiction. The author believes that the catalytic phenomenon is a physical phenomenon rather than a chemical phenomenon. This subject in regard to ECDAM or EODRM will be described in another article in detailed or refer to the article [11] .
As knowledge is limited, the inappropriate ideas are inevitable, so the insight to find out mistakes is welcomed.