Comparison of the Hypoglycemic, Hypolipidemic and Hepatoprotective Effects of Asparagus racemosus Linn. in Combination with Gliclazide and Pioglitazone on Alloxan-Induced Diabetic Rats ()
1. Introduction
Diabetes mellitus (DM) is the most widespread chronic disease of the present decade which is associated with absolute or relative deficiency of circulating insulin level, responsible for persistent high blood glucose level [1] . It turns out either when the beta cells of the pancreas decline to produce a satisfactory amount of insulin hormone or in the situation of produced insulin within the body become resistance [2] . With a significant morbidity and mortality rate, it is acknowledged as a serious metabolic disorder with both the micro-vascular (e.g., retinopathy, nephropathy and neuropathy) and macro-vascular (e.g., myocardial infarction, peripheral vascular disease, atherosclerosis and stroke) complications [3] [4] . DM is addressed as an inaugural cause of death and disability worldwide now-a-days [5] [6] with a global prevalence of about 8% in 2011 and is portended to rise to 10% by 2030 as well as the 7th leading cause of death in the world [7] . People in under-developed and developing countries encompass nearly 80% of the population associated with DM [7] . Mostly affected regions belong to Asia and the eastern Pacific [7] - [12] where in 2011; China had the largest number of adults with diabetes, followed by India and Bangladesh [7] . According to statistics of World Health Organization (WHO), there were around thirty million people who were diagnosed with diabetes worldwide in 1985. This number spiked to 135 million by 1995 and reached up to 217 million in 2005. By the year 2030, WHO forecasts this number will be raised to at least 366 million [13] . This growth in diabetes prevalence is driven principally by an increased prevalence of type 2 diabetes (T2D) in both developing and developed countries [13] . Laterally, the incidence of type 1 diabetes (T1D) is also increasing to that of T2D worldwide [14] [15] [16] .
Due to the abnormal atherogenic profile attributed by elevated very low-den- sity lipoprotein (VLDL) cholesterol, triglycerides (TG) and low density lipoprotein (LDL) cholesterol (small and dense) levels and reduced high density lipoprotein (HDL) cholesterol levels, dyslipidemia originated from T2D exacerbates cardiovascular disease (CVD) risks. Such lipoproteins modification is initiated by oxidation and glycosylation, resulting in an aberration of vascular integrity, disposing to premature and aggressive atherosclerosis [17] . Large numbers of epidemiological study and pathological data established that diabetes is an autonomous risk factor for CVD in both men and women [18] [19] [20] . Most significantly, inherent protection against developing CVD seems to be lost in women with diabetes [18] [21] . CVDs are cataloged as the major element of death in more than 50 percent of patients with diabetes [22] . By the same indication, if the inception of DM does not treat with any lipid-lowering drug over a very lengthy period of time then it revs up the possibilities of the risk of CVD. The treatment of diabetes is not only riddled and tiresome but also pretty expensive which is not affordable for the majority of the African and Asian population [23] . For ensuring a healthy way of living, the contemporary treatments for diabetes mellitus necessitates the use of insulin and synthetic drugs such as sulfonylurea, metformin, alpha-glucosidase inhibitors and thiazolidinedione. Single therapy of any of these drugs is not sufficient to control diabetes related complications accurately [24] [25] . Thus, for controlling blood glucose level and cholesterol level, combination therapy has become undoubtedly well-appreciated [26] [27] . As a result, combination therapy is being regarded as the most efficient remedy, on account of their potentiality to revive the function of the pancreas with an improved insulin output, to slacken the intestinal absorption of glucose, to process the metabolites in insulin dependent pathways [28] and by inhibiting the enzyme HMG-CoA (3-hydroxy-3-methyl-glutaryl-coenzyme A reductase) reductase to cease the cholesterol biosynthetic route [29] .
Asparagus racemosus (AR) Linn. is commonly known in Bengali Shatamuli belongs to Liliaceae family which is indigenous in Bangladesh, India, Asia, Australia and Africa [30] . With numerous succulent roots, AR is spinous under shrub growing at a height of 1500 m. The rhizome of AR is generally used as both conventional medical applications and as a food supplement [31] . The under shrub of AR is typically used for enhancing the activity of immunomodulatory and galactagouge effects according to Ayurveda [32] . Additionally, crude extracts and isolated components of AR have shown a wide range of biological activities, including anti-tumour [33] , antifungal [34] , anti-mutagenic [35] , immunostimulatory [36] [37] [38] and diuretic [39] properties. It also displays immune protective effect in cancer chemotherapy [40] . Roots of AR have been used in the treatment of numerous disorders like nervous disorders, dyspepsia, inflammations, nephropathy, hepatopathy, throat infections, tumors, cough, bronchitis, hyperacidity and diarrhea [41] . Chemically the roots are indicated to contain 9,10-dihydro-1,5-dimehtoxy-8-methyl-2,7-phenanthrenediol [42] , an alkaloid named asparagamine A [43] , and saponins like shatavarin I-IV [44] . The chemical constituents of AR include flavonoids, oligosaccharides, amino acids, sulphur-containing acids and steroidal saponins [45] . Derived polysaccharides are accountable for providing both antioxidant and radioprotective characteristics [46] [47] [48] [49] . Kreskin, one of the derived polysaccharides of AR shows inhibitory activities on the oxidation of low density lipoprotein [47] [48] . The roots of AR have been reported to hold antidiabetic properties by conventional healers. AR plays a pivotal role in lowering of blood glucose level in rats and rabbits [50] [51] . The pharmacological and medical researches for its antidiabetic and hypolipidemic activity are yet to be proved. Studies were done by Hannan et al., [52] , Mahammed et al., [53] and Vadivelan et al., [54] have also claimed the antidiabetic actions of AR.
A preliminary study was shown that ethanolic extracts of AR had antidiabetic and antihyperlipidemic activity in alloxan-induced diabetic animal model [55] . According to the best of our knowledge, this is the first study conducted to determine the combination of antidiabetic drugs with extracts of AR. The current study was designed to compare the hypoglycemic, hepatoprotective and lipid lowering activities of AR root extract alone and in combination with two different classes of antidiabetic drugs in alloxan induced diabetic rats.
2. Materials and Methods
2.1. Drugs and Chemicals
The drugs, gliclazide and pioglitazone were the liberal gift samples from Beximco pharmaceuticals limited, Dhaka, Bangladesh. Alloxan monohydrate was purchased from Explicit Chemicals, Pvt. Ltd, Pune, India. Blood samples analyzed for blood glucose content by using Ez Smart 168 (Tyson Bioresearch, Inc. Chu-Nan, Taiwan) glucose test meter. Total cholesterol (TC), triglyceride (TG), low density lipoprotein (LDL), very low density lipoprotein (VLDL), high density lipoprotein (HDL) kits were purchased from Human Gesellschaft fur Biochemical mbH-Wiesbaden, Germany. Serum glutamate oxaloacetate transaminases (SGOT); Serum glutamate pyruvate transaminases (SGPT) and total protein (TP) kits were purchased from Linear chemicals-Barcelona, Spain. All other chemicals were obtained from local sources and were of analytical grade.
2.2. Collection and Identification of Plant Materials
For this present investigation roots of AR were collected from surrounding area of Kurigram, Bangladesh, in February, 2014. After collection roots were thoroughly washed with water. The plant was identified by expert of Bangladesh National Herbarium, Mirpur, Dhaka, Bangladesh. Accession number: DACB- 39,527 for AR.
2.3. Extraction of Plant Materials
The collected roots were washed and sun dried under a shadow for two weeks. The dried roots were ground into a coarse powder with the help of a suitable grinder. The powdered plant roots having a weight of 500 g was taken in an amber colored glass bottle and soaked in 2.5 liters of 98% ethanol. The bottle was kept at room temperature and allowed to stand for 15 days with occasional shaking and stirring. Then the extracts were filtered through a cotton filter and then through filter paper (Whatman Filter Paper No. 1). Then the liquid filtrates were concentrated and evaporated to dry at temperature 40˚C by using rotary evaporator under reduced pressure to get the crude extract 12.31 g. Finally, the dried crude extracts were stored in a refrigerator at 4˚C until use for the proposed experiment.
2.4. Animals
Healthy, adult male Wister albino rats (140 - 170 g) were purchased from the animal house of Jahangirnagar University, Department of Pharmacy, Savar, Dhaka. The rats were kept in a well-ventilated room and they were exposed to 12 hrs light and 12 hrs night cycle with a temperature between 25˚C ± 2˚C and humidity 60% - 70%. The rats were housed in large spacious, hygienic polypropylene cages during the course of the experimental period. The rats were fed with standard pellet diet, supplied by this same institution. In this study, animals were being used and handled in accordance with the guidelines on animal experimentation of our institute. Every attempt was made to keep the animals in good general health, in conformity with the National Institutes of Health (NIH) [59] . Conduction of this experimental procedures of involved animal complied entirely with the guidelines of the Department of Pharmacy, Southeast University.
2.5. Experimental Design
In this experiment, a total of 50 rats were used. The rats were divided into eight groups and each group contains five rats as follows:
Group 1: Control; only food and water ad libitum were orally administered to rats (Con, n = 5).
Group 2: Diabetic Control; alloxan monohydrate 120 mg/kg b.w. was administered intraperitoneally to rats (DC, n = 5).
Group 3: Diabetic + Plant Extract, 200 mg/kg b.w. (DE200, p.o., n = 5).
Group 4: Diabetic + Plant Extract, 400 mg/kg b.w. (DE400, p.o., n = 5).
Group 5: Diabetic + Gliclazide, 10 mg/kg b.w. (DG, p.o., n = 5).
Group 6: Diabetic + Pioglitazone, 10 mg/70 kg b.w. (DP, p.o., n = 5).
Group 7: Diabetic + Plant Extract, 200 mg/kg b.w. + Gliclazide, 10 mg/kg b.w. (DE200G, p.o., n = 5).
Group 8: Diabetic + Plant Extract, 200 mg/kg b.w. + Pioglitazone, 10 mg/70 kg b.w. (DE200P, p.o., n = 5).
2.6. Preparation of Dosage of Active Drugs and Plant Extract
The solution of gliclazide and pioglitazone was prepared by dissolving with dimethyl sulfoxide (DMSO) and based on literature searches the doses of gliclazide and pioglitazone were selected which were 10 mg/kg b.w. and 10 mg/70 kg b.w. respectively and administered orally to rats [57] [58] . The suspension of EEAR was made by dissolving with normal saline (pH 7.4) and administered orally to rats at 200 and 400 mg/kg b.w and the doses of the EEAR were selected according to the literature searches [55] . Drugs and the suspension of EEAR were prepared freshly everyday.
2.7. Acute Toxicity Study
An acute oral toxicity study was performed for the ethanolic extracts of AR as per guidelines of Organization for Economic Co-operation and Development- 423 guidelines (acute toxic class method) [56] . Healthy male Wister albino rats were randomly divided into six groups with 5 animals in each group used for acute oral toxicity study. The rats were kept fasting overnight with supplementation of water before oral dosing, then the ethanolic extract of AR was administered orally with rising doses (100, 200, 500, 1000, 1500, and 2000 mg/kg b.w.) with the help of an intragastric tube. The rats were closely observed continuously for 24 hrs for behavioral and any others adverse change and subsequently for any lethality.
2.8. Induction of Diabetes
A solution of alloxan monohydrate was prepared by dissolving with normal saline (pH 7.4) and administered by single intra-peritoneal injection (i.p.) to rats in groups 2 to 8 at 120 mg/kg b.w. to induce diabetes, after fasting 16 hrs [60] . After 1 week, measurements of plasma glucose levels were inspected by glucometer using a blood sample from tail-vein of rat. Rats having blood sugar level higher than 11.5 - 13.5 mmol/L were considered as moderate diabetic. A solution of alloxan monohydrate was prepared freshly everyday.
2.9. Antidiabetic Test
In order to determine antidiabetic activity, blood samples were collected from tail-vein of rats and tested for blood glucose content by glucometer at 0th, 5th, 10th and 14th day of treatment.
2.10. Lipid Profile and Hepatic Function Tests
After 14 days of treatment period on 15th day, all rats were anesthetized with diethyl ether then blood samples were collected from the aorta of heart with the help of heparinized syringes and centrifuged by the help of ultra-centrifuge machine (Centurion, UK) at an rpm of 4000 for the time of 20 min to separate serum. There upon, the serum was preserved at −20˚C for scrutinizing TC, TG, LDL-cholesterol, VLDL-cholesterol, HDL-cholesterol, SGOT, SGPT and TP concentrations by using UV spectrophotometric method (Shimadzu UV-1200, Tokyo, Japan) with the aid of wet reagent diagnostic kits according to manufacturer’s protocol.
2.11. Measurement of Body Weight and Organ Weight
The body weight of rats of each group had been recorded on 0th, 5th, 10th and 14th day of treatment period. On 15th day, liver, kidney, pancreases, heart and lung were removed from the sacrificed rats and finally measurement of organ weight was immediately being done. The ratio of organ weights to body weight ratio (O/B) were calculated and stored in 10% formalin, refrigerated at −20˚C.
2.12. Statistical Analysis
Data were expressed as mean ± SEM. Statistical comparison was performed by one-way and two-way (ANOVA) followed by Bonferroni’s multiple comparisons test and the values were considered as statistically significant when p values were less than 0.05 (p < 0.05). Graph pad prism, Version-7 (Graph Pad, Software, Inc.7825 Fay Avenue, Suite 230 La Jolla, CA 92037 USA) and Microsoft Excel 2010 (Roselle, IL, USA) were used for the statistical and graphical evaluations. The results were considered as statistical significance at p < 0.05 compared to disease control group, gliclazide treated group and pioglitazone treated group.
3. Results
3.1. Determination of Acute Toxicity
Oral administration of ethanolic extracts of AR root was showed to be safe up to the dose level of 2000 mg/kg b.w. in rats. In any rat, the extracts did not persuade any toxicological effect.
3.2. Effect of Different Doses of EEAR, Monotherapy of Antidiabetic Drugs and Combination Therapy on Blood Glucose Levels
The effect of different doses of EEAR, monotherapy of antidiabetic drugs and the combination therapy in alloxan-induced diabetic rats were tested for blood glucose lowering activity on 0th, 5th, 10th and 14th day as shown in Figure 1. The administration of EEAR noticeably (p < 0.05, p < 0.01, p < 0.001; p < 0.05, p <
![]()
Figure 1. During 2 weeks of treatment period changes in blood glucose level in normal and alloxan-induced diabetic rats. Here, Con: Control; DC: Diabetic Control; DE200: Diabetic + Plant Extract, 200 mg/kg b.w.; DE400: Diabetic + Plant Extract, 400 mg/kg b.w.; DG: Diabetic + Gliclazide, 10 mg/kg b.w.; DP: Diabetic + Pioglitazone, 10 mg/70 kg b.w.; DE200G: Diabetic + Plant Extract, 200 mg/kg b.w. + Gliclazide, 10 mg/kg b.w.; DE200P: Diabetic + Plant Extract, 200 mg/kg b.w. + Pioglitazone, 10 mg/70 kg b.w. Values were expressed as mean ± SEM (n = 5/group). *p < 0.05, **p < 0.01, ***p < 0.001 significant difference from the disease control group. #p < 0.05, ##p < 0.01, ###p < 0.001 significant difference from the gliclazide treated group. +p < 0.05, ++p < 0.01, +++p < 0.001 significant difference from the pioglitazone treated group.
0.01; p < 0.05) reduced the blood glucose levels in rats on 10th and 14th day of treatment in a dose-dependent mood when compared to that of disease control, gliclazide treated and pioglitazone treated rats. The effect of combination therapy in rats was significantly (p < 0.001; p < 0.01, p < 0.001; p < 0.05, p < 0.01, p < 0.001) lowered the blood glucose levels on 5th, 10th and 14th day of treatment as compared to the disease control, gliclazide treated and pioglitazone treated rats.
3.3. Effect of Different Doses of EEAR, Monotherapy of Antidiabetic Drugs and Combination Therapy on Lipid Profile
The serum TG, TC, LDL and VLDL cholesterol levels were significantly higher in the disease control rats, while the HDL cholesterol levels were significantly decreased in the disease control rats (Figure 2). The effect of EEAR in rats considerably improved (p < 0.05, p < 0.01, p < 0.001; p < 0.05, p < 0.01; p < 0.05) the activity of TC, TG, LDL, VLDL and HDL cholesterol levels in a dose-dependent approach with respect to that of disease control, gliclazide treated and pioglitazone treated rats. The administration of combination therapy also significantly ameliorated (p < 0.001; p < 0.01, p < 0.001) hyperlipidemic condition in terms of TG, LDL and HDL cholesterol levels with insignificant alters in VLDL and TC level while comparing with group receiving monotherapy of antidiabetic drugs, gliclazide treated and pioglitazone treated rats.
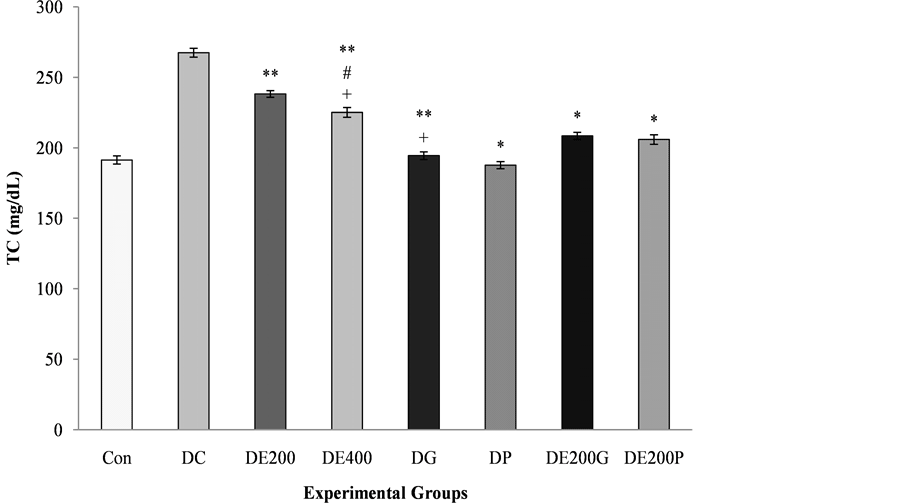
(a)

(b)
![]() (c)
(c)![]() (d)
(d)![]() (e)
(e)
Figure 2. Hypolipidemic effect of EEAR, monotherapy of antidiabetic drugs and combination therapy on lipid profile in alloxan-induced diabetic rats. Here, Con: Control; DC: Diabetic Control; DE200: Diabetic + Plant Extract, 200 mg/kg b.w.; DE400: Diabetic + Plant Extract, 400 mg/kg b.w.; DG: Diabetic + Gliclazide, 10 mg/kg b.w.; DP: Diabetic + Pioglitazone, 10 mg/70 kg b.w.; DE200G: Diabetic + Plant Extract, 200 mg/kg b.w. + Gliclazide, 10 mg/kg b.w.; DE200P: Diabetic + Plant Extract, 200 mg/kg b. w. + Pioglitazone, 10 mg/70 kg b.w. Values were expressed as mean ± SEM (n = 5/group). (a) Total cholesterol; (b) Triglycerides; (c) Low density lipoprotein; (d) Very low density lipoprotein; (e) High density lipoprotein. *p < 0.05, **p < 0.01, ***p < 0.001 significant difference from the disease control group. #p < 0.05, ##p < 0.01, ###p < 0.001 significant difference from the gliclazide treated group. +p < 0.05, ++p < 0.01, +++p < 0.001 significant difference from the pioglitazone treated group.
3.4. Effect of Different Doses of EEAR, Monotherapy of Antidiabetic Drugs and Combination Therapy on Hepatic Function
The levels of hepatic enzymes such as SGOT, SGPT and TP levels were significantly higher in the disease control rats given in Figure 3. The rats treated with EEAR noticeably (p < 0.05, p < 0.01, p < 0.001; p < 0.05, p < 0.01; p < 0.05, p < 0.01) reduced the liver enzymes level including SGOT, SGPT and TP in a dose-dependent manner as compared to that of disease control, gliclazide treated and pioglitazone treated rats. The effect of combination therapy markedly (p <
![]() (a)
(a)![]() (b)
(b)![]() (c)
(c)
Figure 3. Hepatoprotective effect of different doses of EEAR, monotherapy of antidiabetic drugs and combination therapy on hepatic enzyme markers in alloxan-induced diabetic rats. Here, Con: Control; DC: Diabetic Control; DE200: Diabetic + Plant Extract, 200 mg/kg b.w.; DE400: Diabetic + Plant Extract, 400 mg/kg b.w.; DG: Diabetic + Gliclazide, 10 mg/kg b.w.; DP: Diabetic + Pioglitazone, 10 mg/70 kg b.w.; DE200G: Diabetic + Plant Extract, 200 mg/kg b.w. + Gliclazide, 10 mg/kg b.w.; DE200P: Diabetic + Plant Extract, 200 mg/kg b. w. + Pioglitazone, 10 mg/70 kg b.w. Values were expressed as mean ± SEM (n = 5/group). (a) Serum glutamate oxaloacetate transaminases; (b) Serum glutamate pyruvate transaminases; (c) Total protein. *p < 0.05, **p < 0.01, ***p < 0.001 significant difference from the disease control group. #p < 0.05, ##p < 0.01, ###p < 0.001 significant difference from the gliclazide treated group. +p < 0.05, ++p < 0.01, +++p < 0.001 significant difference from the pioglitazone treated group.
0.001; p < 0.001; p < 0.01, p < 0.001) decreased the SGOT, SGPT and TP hepatic enzyme levels when compared to the disease control, gliclazide treated and pioglitazone treated rats indicated improvement in liver dysfunctions.
3.5. Determination of Survival Rate
The survival rate in all the experimental groups of rats is shown in Table 1. After 2 weeks of treatment it was observed that the survival rate among the EEAR treated rats was identical (DE200 = 60%, DE400 = 60%). The minimal survival rate was found among the disease control rats (DC = 40%). None of the rats died in combination therapy treated rats and control rats. After 2 weeks of treatment period, the maximum survival rate was found in combination therapy treated rats (100%).
3.6. Effect of Different Doses of EEAR, Monotherapy of Antidiabetic Drugs and Combination Therapy on Body Weight Changes and Organ Weight
Body weight changes in all the experimental groups of rats were shown in Table 2. The body weight of disease control rats showed significantly decreased during 2 weeks of treatment period. During treatment period it was observed that the considerable changes (p < 0.001; p < 0.01, p < 0.001; p < 0.05) in the body weight were found in EEAR treated rats and combination therapy treated rats on 10th and 14th day of treatment when compared to that of disease control, gliclazide treated and pioglitazone treated rats. The weight of heart, liver, lung, pancreas and kidney did not noticeably changes after 2 weeks of treatment in all the experimental groups of rats (Table 3). Although the weight of liver and pancreas meaningfully decreased in disease control rats, after 2 weeks of treatment the values were controlled (p < 0.001; p < 0.05, p < 0.01; p < 0.05, p < 0.01, p < 0.001)
![]()
Table 1. Effect of different doses of EEAR, monotherapy of antidiabetic drugs and combination therapy on the survival rate of experimental rats after 2 weeks of treatment period.
Here, Con: Control; DC: Diabetic Control; DE200: Diabetic + Plant Extract, 200 mg/kg b.w.; DE400: Diabetic + Plant Extract, 400 mg/kg b.w.; DG: Diabetic + Gliclazide, 10 mg/kg b.w.; DP: Diabetic + Pioglitazone, 10 mg/70 kg b.w.; DE200G: Diabetic + Plant Extract, 200 mg/kg b.w. + Gliclazide, 10 mg/kg b.w.; DE200P: Diabetic + Plant Extract, 200 mg/kg b.w. + Pioglitazone, 10 mg/70 kg b.w. Values of the survival rate were expressed as percentages, % (n = 5/group).
![]()
Table 2. Effect of different doses of EEAR, monotherapy of antidiabetic drugs and combination therapy on body weight changes of experimental rats during the treatment period.
Here, Con: Control; DC: Diabetic Control; DE200: Diabetic + Plant Extract, 200 mg/kg b.w.; DE400: Diabetic + Plant Extract, 400 mg/kg b.w.; DG: Diabetic + Gliclazide, 10 mg/kg b.w.; DP: Diabetic + Pioglitazone, 10 mg/70 kg b.w.; DE200G: Diabetic + Plant Extract, 200 mg/kg b.w. + Gliclazide, 10 mg/kg b.w.; DE200P: Diabetic + Plant Extract, 200 mg/kg b.w. + Pioglitazone, 10 mg/70 kg b.w. Values were expressed as mean ± SEM (n = 5/group) and the unit of measurement was gram (g). ***p < 0.001 significant difference from the disease control group. ##p < 0.01, ###p < 0.001 significant difference from the gliclazide treated group. +p < 0.05 significant difference from the pioglitazone treated group.
![]()
Table 3. Effect of different doses of EEAR, monotherapy of antidiabetic drug and combination therapy on organ weight of experimental rats after 2 weeks of treatment period.
Here, Con: Control; DC: Diabetic Control; DE200: Diabetic + Plant Extract, 200 mg/kg b.w.; DE400: Diabetic + Plant Extract, 400 mg/kg b.w.; DG: Diabetic + Gliclazide, 10 mg/kg b.w.; DP: Diabetic + Pioglitazone, 10 mg/70 kg b.w.; DE200G: Diabetic + Plant Extract, 200 mg/kg b.w. + Gliclazide, 10 mg/kg b.w.; DE200P: Diabetic + Plant Extract, 200 mg/kg b.w. + Pioglitazone, 10 mg/70 kg b.w. Values were expressed as mean ± SEM (n = 5/group) and the unit of measurement was gram (g). ***p < 0.001 significant difference from the disease control group. #p < 0.05, ##p < 0.01 significant difference from the gliclazide treated group. +p < 0.05, ++p < 0.01, +++p < 0.001 significant difference from the pioglitazone treated group.
in the highest dose of EEAR (DE400) treated rats and combination therapy treated rats as compared to the disease control, gliclazide treated and pioglitazone treated rats.
4. Discussion
Among the all chronic diseases, diabetes mellitus is the most trivial and affiliated with hyperlipidemia and co-morbidities such as obesity, hypertension [61] [62] [63] . Hyperlipidemia is marked as a serious kind of intricacy for both clinical and experimental diabetes due to the subsequent metabolic complications [64] . Severe hyperglycemia is the most prominent reason for the complications of DM. Chronic complications are characterized by the dysfunction of various organs, especially the eyes (retinopathy), kidneys (nephropathy), nerves (neuropathy), heart (cardiomyopathy) and blood vessels [65] [66] .
Effect of different doses of EEAR groups and the combination groups has been evaluated for its antidiabetic, antihyperlipidemic and hepatoprotective potential in this current study. A potent diabetogenic named alloxan is reduced to dialuric acid which is auto-oxidized back to alloxan afterward resulting in the production of H2O2, O2, 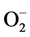 and OH radicals and genesis damages to the beta-cells of islets of Langerhans [67] . Meanwhile, there is a humongous dwindling in insulin level and the consequent increase in fasting blood glucose level [68] . It consequences poor glycemic control and excessive catabolism of protein to provide amino acids for gluconeogenesis during insulin deficiency triggering is polyuria, polyphagia and body weight reduction [69] .
and OH radicals and genesis damages to the beta-cells of islets of Langerhans [67] . Meanwhile, there is a humongous dwindling in insulin level and the consequent increase in fasting blood glucose level [68] . It consequences poor glycemic control and excessive catabolism of protein to provide amino acids for gluconeogenesis during insulin deficiency triggering is polyuria, polyphagia and body weight reduction [69] .
In the present study, treatment with EEAR in male Wister albino rats produced significant glucose lowering activity when compared to disease control rats and monotherapy of antidiabetic drug rats. Hannan et al., reported that the extract of Asparagus racemosus roots improved insulin secretion from perfusing pancreas, isolated islets and clonal pancreatic β-cells [30] . In our study, after 2 weeks of treatment it was found that combination of EEAR with gliclazide and pioglitazone produced a significant decrease in the blood glucose level which was greater than that produced by monotherapy of antidiabetic drugs. Therefore it looks like that, rather than its pancreatic action, EEAR may also belong to extrapancreatic action, which could contribute to its hypoglycemic action. In the study on the effect of Shilajit on blood glucose and lipid profile in alloxan induced diabetic rats by Trivedi et al., reported almost analogous findings [70] .
The alloxan-induced diabetic rats showed a remarkable increase in the levels of lipid profiles such as TG, TC and lipoproteins including LDL-cholesterol, VLDL-cholesterol and marked the reduction in HDL-cholesterol [71] . In conditions of diabetes, a multifarious number of studies have demonstrated that there is an alteration of lipid profiles indicating a risk factor for CVD [72] [73] . Ghoul et al., has revealed that insufficiency in insulin or the insulin resistance may be accountable for dyslipidemia on account of the insulin inhibiting action on the major enzyme in the cholesterol biosynthesis [74] . High levels of TC and more importantly LDL-cholesterol in blood are dominant coronary risk factors. Since insulin inhibits the hormone sensitive lipase and so in diabetic condition, the lack of insulin causes the abnormally high concentrations of serum lipids due to the increase in the mobilization of free fatty acids from the peripheral fat depots [75] [76] [77] . A key rate-limiting the enzyme responsible for the metabolism of cholesterol-rich LDL particles are HMG-CoA reductase upon which insulin has an inhibitory action that is why insulin deficiency or insulin resistance is responsible for dyslipidemia [78] . From our present study demonstrated that, after 2 weeks of treatment oral administration of adjunct therapy was remarkably improved the hyperlipidemic condition in case of TG, LDL and HDL cholesterol levels with insignificant changes in VLDL and TC level when compared to monotherapy of antidiabetic drug. EEAR treated rat groups showed favorable effects on the lipid profile in male Wister albino rats by lowering TG, LDL, VLDL and TC and increasing HDL meaningfully as compared to the disease control rats and monotherapy of antidiabetic drug. Therefore, it is predictable that EEAR encouraged favorable changes in the lipid profile in diabetic rats that is may not only due to better glycemic control, but could also be due to its first- hand action on lipid metabolic pathways. In the study of Bersama engleriana on the lipid profile of rats by Watcho et al., also claimed similar outcomes [79] .
Since the liver is the fundamental metabolic organ in body accountable for the homeostasis of the glucose and fat, diabetes causes to raise hepatic dysfunction. Alloxan induced diabetic rats promoted hepatic damage that leads to the rise in the enzyme activities [80] . The evaluations of hepatic abnormalities are corroborated by increasing in serum enzymes including SGPT, SGOT and TP [81] . In disease control group, an increase in these enzyme activities reflects active liver damage. Extremely elevated transaminase levels are also observed in an account of inflammatory hepatocellular disorders [82] . Liver necrosis in alloxan-induced diabetic rats augmented in the activities of SGPT, SGOT and TP in plasma might be mainly due to the leakage of these enzymes from the liver cytosol into the bloodstream [83] . On the contrary, reduction in the activity of these enzymes in plasma when compared to the diabetic control group and consequently alleviated liver damage is taken during the treatment of the experimental rats with different doses of EEAR, monotherapy of antidiabetic drug and the combination of antidiabetic drugs and plant extract. Our investigation found that, after 2 weeks of treatment of EEAR showed remarkably reduced the levels of SGPT, SGOT and TP in plasma as compared to disease control ratsand monotherapy of antidiabetic drug. The effect of combination treatment was significantly decreased the levels of SGPT, SGOT and TP liver enzyme levels when compared to the disease control rats and monotherapy of antidiabetic drug indicated improvement in liver dysfunctions. Toma et al., in the study on Moringa stenopetala leaves extracts in rats claimed considerably reduced in the hepatic enzymes levels that cause liver damage [84] .
The present observation found that none of the rats died in combination group. The survival rate was significantly higher in combination therapy treated rats when compared to the disease control rats. In the study on effect of nuts displayed no mortality when administered in combinations with Emblica officinalis and honey as indicated by Rajendran et al., [85] .
No significant changes in the body weight and organ weight to body weight ratio were observed among the treatment groups. Although the weight of liver and the weight of pancreas significantly decreased, the weight of heart, kidney and lung did not change after 2 weeks of treatment. Treatment with combination therapy treated rats and highest dose of EEAR (DE400) considerably improved liver weight and pancreas weight when compared to the disease control rats and monotherapy of antidiabetic drugs.
5. Conclusion
The progression of diabetes has become an issue of great concern globally and is being considered as a life-threatening disorder for the human being. Although numerous treatments are available to treat this devastating illness, most of them possess adverse effects. As a result, scientists are now searching natural remedies to treat this disease. As a part of this endeavor current investigation aimed to determine the beneficial effects of root extract of AR alone and in combination with antidiabetic drugs (gliclazide and pioglitazone). The outcome of this research indicated that the combination therapy delivered synergistic effect on reducing blood glucose level in the controlling of diabetes than that of monotherapy of antidiabetic drugs. The combination therapy also considerably ameliorated hyperlipidemic condition in terms of TG, LDL and HDL cholesterol levels with insignificant alter in VLDL and TC level when compared to monotherapy of antidiabetic drug. They also improved liver dysfunctions by decreasing the hepatic enzyme levels, including SGOT, SGPT and TP. Further investigation is required to characterize the compounds that are particularly responsible for their beneficial effects and to determine their mechanism of action.
Acknowledgements
The authors wish to thank the Department of Pharmacy, Southeast University, Dhaka, Bangladesh for providing research facilities.
Authors’ Contributions
This work was carried out in collaboration among all authors. AFMTR: Designed the study, wrote the protocol and managed the analyses of the study. AAM, MH and MSU: Carried out the laboratory tests and prepared the draft of the manuscript. MTI, SH and MFH: Prepared the plant extracts and managed the literature searches. AAM, MSH, ARS and MMR: Performed statistical and graphical evaluations. MR and MMR: Reviewed the scientific contents of the manuscript. All the authors read and approved the final manuscript.
Ethical Approval
The protocol of the experiment was approved by the animal ethics committee of the Department of Pharmacy, Southeast University, Dhaka, Bangladesh. The animals care and health were maintained according to the guidelines of NIH.
Competing Interests
The authors proclaim that there is no competing interests exist about the content of this article.
Abbreviations
DM: Diabetes mellitus;
T1D: Type 1 diabetes;
T2D: Type 2 diabetes;
CVD: Cardiovascular diseases;
WHO: World Health Organization;
HMG-CoA: (3-hydroxy-3-methyl-glutaryl-coenzyme A reductase;
DMSO: Dimethyl sulphooxide;
AR: Asparagus racemosus;
EEAR: Ethanolic Extract of Asparagus racemosus;
b.w.: Body weight;
p.o.: Per os (by mouth);
hrs: Hours;
TC: Total cholesterol;
TG: Triglycerides;
LDL: Low density lipoprotein;
VLDL: Very low density lipoprotein;
HDL: High density lipoprotein;
SGOT: Serum glutamate oxaloacetate transaminases;
SGPT: Serum glutamate pyruvate transaminases;
TP: Total protein;
Con: Control;
DC: Diabetic Control;
DE200: Diabetic + Plant Extract, 200 mg/kg b.w.;
DE400: Diabetic + Plant Extract, 400 mg/kg b.w.;
DG: Diabetic + Gliclazide, 10 mg/kg b.w.;
DP: Diabetic + Pioglitazone, 10 mg/70 kg b.w.;
DE200G: Diabetic + Plant Extract, 200 mg/kg b.w. + Gliclazide, 10 mg/kg b.w.;
DE200P: Diabetic + Plant Extract, 200 mg/kg b.w. + Pioglitazone, 10 mg/70 kg b.w.