Enhanced Visible-Light-Sensitive Two-Step Overall Water-Splitting Based on Band Structure Controls of Titanium Dioxide and Strontium Titanate ()
1. Introduction
Various photocatalytic materials have been evaluated for water splitting activity because the generated hydrogen (H2) represents a clean and renewable fuel source [1] [2] [3] . Among examined materials, titanium dioxide (TiO2) with which Fujishima and Honda first demonstrated photoinduced water-splitting [1] and strontium titanate (SrTiO3) [4] , are the most promising photocatalysts due to their abundance, nontoxicity, thermal stability and high resistance against photo-corrosion. Despite these advantageous properties, both TiO2 and SrTiO3 are only sensitive to ultraviolet (UV) light and therefore requires modification for the utilization of visible light. To this end, numerous studies have examined the effects of doping foreign elements into TiO2 and SrTiO3 on visible-light induced water-splitting [5] [6] [7] [8] . Although a sacrificial agent is needed, the doped TiO2 and SrTiO3 are able to generate either H2 or oxygen (O2) following irradiation with visible light (half reaction of water-splitting).
Combined systems consisting of two specific photocatalysts for H2 and O2 production (H2-photocatalyst and O2-photocatalyst, respectively) and a suitable redox mediator can function as visible-light sensitive photocatalysts for overall water-splitting (termed two-step overall water-splitting or Z-scheme overall water-splitting) [9] - [18] . The well-known combination is platinum (Pt)-deposited chromium (Cr) and tantalum (Ta)-codoped SrTiO3 or ruthenium (Ru)-deposited rhodium (Rh)-doped SrTiO3 as the H2-photocatalyst, and Pt-deposited tungsten trioxide (WO3) or bismuth vanadate (BiVO4) as the O2-photocatalyst [10] [11] . The Z-scheme systems require the suitable redox mediator, such as iodate (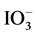 )/iodide (I−) or ferric (Fe3+)/ferrous (Fe2+) ions. Recently, solid-state Z- scheme systems that function in the absence of a redox mediator have been reported [13] - [18] .
)/iodide (I−) or ferric (Fe3+)/ferrous (Fe2+) ions. Recently, solid-state Z- scheme systems that function in the absence of a redox mediator have been reported [13] - [18] .
Our group have successfully synthesized a combined system consisting of only one mother material, TiO2-based photocatalysts (Pt-deposited anatase Ti0.96Cr0.02Ta0.02O2 (Pt/Ti0.96Cr0.02Ta0.02O2) and Pt-deposited rutile Ti0.982Cr0.009Ta0.009O2 (Pt/Ti0.982Cr0.009Ta0.009O2) as the H2- and O2-photocatalyst, respectively) and SrTiO3-based photocatalysts (Ru-deposited Sr(Ti0.99Rh0.01)O3 and Ru-deposited (Sr0.99Na0.01)(Ti0.99V0.01)O3, as the H2- and O2-photocatalyst, respectively) [19] [20] . Based on the activities of the systems, it was conceivable that visible-light- sensitive two-step overall water-splitting system could be improved by utilizing the SrTiO3- and TiO2-based materials as H2- and O2-photocatalysts, respectively. The construction of such a system may be advantageous because they have properties, such as nontoxicity, stability, and natural abundance, which are expected to facilitate their use in industrial and practical applications by contrast with utilization of WO3 or BiVO4.
In the present study, we combined Cr- and Ta-codoped TiO2 and Rh-doped SrTiO3, and achieved the H2- and O2-evolution rates derived from two-step overall watersplitting ~100 times larger irradiated with 420-nm visible-light.
2. Experimental Section
2.1. Preparations of TiO2-Based and SrTiO3-Based Photocatalysts
Cr- and Ta-codoped rutile TiO2 (Ti0.986Cr0.007Ta0.007O2, Cr,Ta-TiO2) as an O2- evolution photocatalyst was prepared by a hydrothermal synthesis method using commercial Ti(SO4)2 (24.0% purity, 3.97 × 10−2 mol; Kanto Kagaku), CrCl3・6H2O (1.40 × 10−4 mol; Kanto Kagaku), and TaCl5 (1.40 × 10−4 mol; Kanto Kagaku) as starting materials. The starting materials for the form of Cr,Ta-TiO2 were mixed and stirred in distilled water for 30 min using a magnetic stirrer. The solutions were treated hydrothermally in an autoclave at 140˚C for 12 h, and the resulting mixtures were washed with sufficient distilled water, collected by centrifugation, and dried at 80˚C overnight. The dried mixtures were calcined at 900˚C for 24 h, and were then thoroughly ground using a mortar and pestle. As a reference, non-doped rutile (TiO2) was prepared under identical conditions using only Ti(SO4)2.
Rh-doped SrTiO3 (Sr(Ti0.99Rh0.01)O3, Rh-SrTiO3) was synthesized using a con- ventional solid-state reaction. Commercial SrCO3 (Kanto Kagaku, purity 99.9%), TiO2 (High Purity Chemicals, purity 99.99%), and Rh2O3 (Kanto Kagaku, purity 99.9%) powders were used as the starting materials. Stoichiometric amounts of the starting materials for Rh-SrTiO3 were wet-ball-milled (200 rpm) for 20 h using zirconium dioxide (ZrO2) balls as the milling medium in polyethylene bottles. The resulting mixture was calcined at 1000˚C for 10 h and then thoroughly ground to obtain Rh-SrTiO3 powder. As a reference, non-doped SrTiO3 was prepared under identical conditions using only SrCO3 and TiO2.
The deposition of either Pt or Ru co-catalyst onto the synthesized Cr,Ta-TiO2 or Ru-SrTiO3 photocatalysts, respectively, was performed by a photo-deposition method. Briefly, 0.5 g of either Cr,Ta-TiO2 or Ru-SrTiO3 powder was first dispersed in 100 mL methanol solution (20 vol%) as a hole scavenger. The amount of H2PtCl6・6H2O (98.5% purity; Kanto Kagaku) as the source of Pt needed to give a weight fraction of Pt relative to Cr,Ta-TiO2 of 1 × 10−3 was weighted. Ruthenium chloride (RuCl3∙nH2O, n was assumed to be 3), which served as the source of Ru, was weighed to give a weight fraction of Ru relative to Rh-SrTiO3 of 7 ´ 10−3. Weighed H2PtCl6・6H2O or RuCl3∙nH2O was added to each aqueous sample suspension, which was then sufficiently deaerated using liquid nitrogen (N2). While deaerating the suspensions, a xenon (Xe) lamp (LA-251 Xe; Hayashi Tokei) equipped with an optical filter (Y-44, Hoya) was employed for light irradiation of the suspension for 4 h. The suspension was then centrifuged, washed with distilled water, and the resulting residues were dried at 80˚C overnight. The residues were ground into a fine powder using an agate mortar to obtain the Pt-deposited Cr,Ta-TiO2 (Pt/Cr,Ta-TiO2) and Ru-deposited Rh-SrTiO3 (Ru/Rh-SrTiO3) photocatalyst powders.
2.2. Characterizations
The crystal structures of the prepared powders were examined by X-ray diffraction (XRD) using a PW-1700 system (Panalytical). Brunauer-Emmett-Teller (BET) surface areas were determined using a nitrogen adsorption apparatus (Micromeritics, TriStar 3000; Shimadzu). The valency of the constituent elements was measured by X-ray photoelectron spectroscopy (XPS; Axis-Ultra, Shimadzu). UV-visible absorption spectra were obtained by the diffuse reflection method using a V-650 (JASCO) spectrometer. Quantitative analyses were performed by X-ray fluorescence (XRF) using a ZSXP PrimusII system (Rigaku). A scanning transmission electron microscope (STEM, Tecnai Osiris, FEI) were used to observe the morphology of the prepared photocatalysts.
2.3. Photocatalytic Water-Splitting Tests
Two types of photocatalytic water-splitting tests (half reactions of water-splitting and two-step overall water-splitting) were performed in a gas-closed-circulation system, which was filled with argon gas (50 kPa) after deaeration. The amounts of evolved H2 and O2 were monitored using an online gas chromatograph (GC-8A; Shimadzu). Each time we performed the water-splitting experiments, a different amount of N2 was detected. We repeatedly deaerated this system to a final pressure of 2.5 Pa and then introduced argon gas into the system in the same way. For these reasons, we considered that the detection of N2 originated from the intruded air from outside, and the effect of residual O2 (and N2) in water was possibly excluded [19] [20] [21] . Thus, if N2 was detected, the O2 amount was calculated using the following equation: O2 = obs. O2 ? (obs. N2/0.78) × 0.21.
H2 and O2 evolution derived from the half reaction of water-splitting was monitored in the presence of Ru/Rh-SrTiO3 or Pt/Cr,Ta-TiO2 (60 mg each) with the aid of iodide ion (I−) (sodium iodide (NaI), 99.5% purity, 0.01 mol/L Kanto Kagaku) or iodate ion (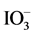 ) (sodium iodate (NaIO3), 99.5%purity, 0.01 mol/L; Kanto Kagaku), respectively, as a sacrificial agent. The examinations were conducted in 10 mL of solution, without adjusting the pH, with constant stirring using a magnetic stirrer and under irradiation with visible light generated from a light-emitting diode (LED) lamp with a wavelength of 420 nm (420 nm-LED, LEDH60-420, Hamamatsu Photonics). Two-step overall water-splitting experi- ments were conducted by adding the sample powders (Ru/Rh-SrTiO3: 10 mg, Pt/Cr,Ta-TiO2: 50 mg) to 10 mL
) (sodium iodate (NaIO3), 99.5%purity, 0.01 mol/L; Kanto Kagaku), respectively, as a sacrificial agent. The examinations were conducted in 10 mL of solution, without adjusting the pH, with constant stirring using a magnetic stirrer and under irradiation with visible light generated from a light-emitting diode (LED) lamp with a wavelength of 420 nm (420 nm-LED, LEDH60-420, Hamamatsu Photonics). Two-step overall water-splitting experi- ments were conducted by adding the sample powders (Ru/Rh-SrTiO3: 10 mg, Pt/Cr,Ta-TiO2: 50 mg) to 10 mL 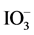 solution as a starting redox mediator without I−. The suspension was constantly stirred using a magnetic stirrer and the pH was not adjusted. The 420 nm-LED light was used for light irradiation.
solution as a starting redox mediator without I−. The suspension was constantly stirred using a magnetic stirrer and the pH was not adjusted. The 420 nm-LED light was used for light irradiation.
3. Results and Discussion
3.1. Characterization of the Prepared Photocatalysts
Elemental analysis by XRF indicated that the molar ratio of Ti:Cr:Ta in Pt/Cr,Ta-TiO2 was 0.994:0.002:0.004. Notably, this molar ratio was not consistent with the starting ratios used in the preparation of the photocatalyst. This discrepancy was attributed to differences in the solubility of Ti, Cr and Ta in aqueous solution under hydrothermal conditions. The analysis also indicated that the molar fraction of Pt relative to Cr,Ta-TiO2 was 1 ´ 10−4. The analysis indicated that the molar ratios of Sr:Ti:Rh in Rh/Rh-SrTiO3 was 0.493:0.493:0.014 nearly equal to the starting fractions used in the preparation. The Ru amount relative to Rh-SrTiO3 was observed to be 1 ´ 10−2.
Cr,Ta-TiO2 was confirmed to have a single phase of rutile TiO2, in the ob- tained XRD spectrum (Figure 1(a)). In addition, the XRD peaks of Cr,Ta-TiO2 were shifted to a lower 2θ angle compared to non-doped TiO2 (Figure 1(b)). These results are reasonable when one considers that the effective ionic radii of Ti4+, Cr3+, and Ta5+ (six-coordination) are 0.0605, 0.0615, and 0.064 nm,
![]()
Figure 1. XRD patterns of prepared TiO2 and Cr,Ta-TiO2 powders (a), and SrTiO3 and Rh-SrTiO3 (c). (b) and (d) are enlargements of (a) and (c), respectively.
respectively [22] . Thus, in the rutile form of the TiO2 photocatalyst, Cr and Ta ions were incorporated at Ti sites. The XRD pattern of the synthesized Rh-SrTiO3 powder indicated the photocatalyst formed a cubic crystal structure with a perovskite crystalline phase (Figure 1(c)). In Figure 1(d), the peak of Rh-SrTiO3 shifted to a lower 2θ angle compared to non-doped SrTiO3. According to Konda et al. [23] , two different species of Rh (Rh3+ (0.0665 nm, the effective ionic radius) and a Rh species with a higher oxidation state than Rh3+, such as Rh4+ (0.0615 nm, the same)) replaced Ti sites as dopants. Thus, we considered that Rh ions were incorporated at Ti sites in SrTiO3.
XPS spectra were recorded to confirm the valency of constituent ions in Cr,Ta-TiO2 and Rh-SrTiO3. Figures 2(a)-2(d) show the spectra for the Ti 2p, Cr 2p, Ta 4f, and O1s orbitals, respectively, of the prepared TiO2, Cr,Ta-TiO2, and commercially available TiO2 (99% purity, Kanto Kagaku) after calibration with the C 1s peak, derived from a conductive carbon-tape that had a binding energy of 284.5 eV. The spectra of Ti 2p of the prepared TiO2 and Cr,Ta-TiO2 were quite similar without any shoulder or peak at lower binding energy side (Figure 2(a)). Thus, the valency of Ti was 4+ in the prepared TiO2 and Cr,Ta-TiO2 [24] [25] . In contrast, the commercial TiO2 had the peaks at lower binding energy side, indicating that it includes Ti3+ [24] . The Cr 2p peaks derived from Cr3+ (Cr 2p3/2 at 576.9 eV and Cr2p1/2 at 587.0 eV [24] ) and Ta 4f peaks from Ta5+ (Ta 4f7/2 at 26 eV and Ta 4f5/2 at 28 eV [26] ) were observed only in Cr,Ta-TiO2 as shown in Figure 2(b) and Figure 2(c), respectively. In Figure 2(d), the O 1s spectra are shown, however, they contain the spectra originated from the carbon tape. So, we are unable to discuss the valency of O and oxygen defects from these spectra. However, we consider that the prepared TiO2 and Cr,Ta-TiO2 do not have much oxygen defects because both of them contain Ti as Ti4+ and Cr,Ta-TiO2 has Cr and Ta as Cr3+ and Ta5+.
![]()
Figure 2. XPS spectra for (a) Ti 2p, (b) Cr 2p, (c) Ta 4f, and (d) O 1s of the prepared TiO2, Cr,Ta-TiO2, and commercial TiO2.
Figures 3(a)-3(d) show the spectra for the Sr 3d, Ti 2p, Rh 3d, and O1s orbitals, respectively, of the prepared SrTiO3, Rh-SrTiO3, and commercially available SrTiO3 (99% purity, Aldrich) after calibration with the C 1s peak similarly. The Sr 3d spectrum peak positions (Sr3d5/2 at 132 eV and Sr 3d3/2 at 134 eV [27] ) were quite similar among the prepared SrTiO3, Rh-SrTiO3, and the commercial SrTiO3. However, the spectrum shape of Rh-SrTiO3 was different from those of both types of SrTiO3 (Figure 3(a)). According to Ehre et al., the Sr 3d spectrum peak positions shift to higher energy region when SrTiO3 becomes amorphous [27] . So, the Sr-O bonding in Rh-SrTiO3 would be looser than that in SrTiO3. As for Ti 2p, Rh-SrTiO3 had shoulders at higher energy side (Figure 3(b)), indicating the existence of the structure similar to Ti3O5 [28] . So, the oxygen defects were presumably incorporated in Rh-SrTiO3. The Rh3d peaks were observed only in Rh-SrTiO3 as shown in Figure 3(c). In Figure 3(d), the observed O 1s spectra contain those originated from the carbon tape. So, we are unable to discuss the valency of O and oxygen defects from these spectra. However, as mentioned above, Rh-SrTiO3 possibly contains oxygen defects.
The UV-visible absorption spectra of TiO2, Cr,Ta-TiO2, Pt/Cr,Ta-TiO2, SrTiO3, Rh-SrTiO3, and Ru/Rh-SrTiO3 are shown in Figure 4. In the spectrum of Cr,Ta-TiO2, an absorption shoulder in the visible-light region and a negligible shift of the absorption edges were observed compared to that of TiO2. These findings indicate that the band-gap of Cr,Ta-TiO2 did not markedly differ from that of TiO2. In contrast, absorption in the entire range of visible light was observed for Rh-SrTiO3, with a peak occurring at ~600 nm. Konda et al. reported the existence of two different doped Rh species as mentioned above is responsible for this spectral pattern [23] . The absorption with a peak occurring at ~600 nm
![]()
Figure 3. XPS spectra for (a) Sr 3d, (b) Ti 2p, (c) Rh 3d, and (d) O 1s of the prepared SrTiO3, Rh-SrTiO3, and commercial SrTiO3.
![]()
Figure 4. UV-visible absorption spectra of TiO2, Cr,Ta-TiO2, Pt/Cr,Ta-TiO2, SrTiO3, Rh-SrTiO3and Ru/Rh-SrTiO3.
originated from the higher oxidation state of the Rh species, and the one with shorter wavelength region from Rh3+. Also, they asserted that the band-gap of Sr(Ti0.99Rh0.01)O3 (i.e., Rh-SrTiO3 in the present study) is narrowed by the partial overlap of Rh 4d6 (Rh3+) and O 2p orbitals to form the VB, resulting in a negative-potential shift of the VB top, which we confirmed in our previous study [19] . After depositing either Pt or Ru onto Cr,Ta-TiO2 or Rh-SrTiO3, respectively, the absorption over a wider wavelength region (>400 nm) clearly increased, indicating the successful deposition of Pt or Ru.
STEM imaging of Pt/Cr,Ta-TiO2 and Ru/Rh-SrTiO3 are shown in Figure 5(a) and Figure 5(b), respectively. The particle sizes of Cr,Ta-TiO2 and Rh-SrTiO3 were estimated to have sizes of ~100 nm and ~50 nm, respectively. The difference in particle sizes reflected the BET surface areas, which were observed to be 1.4 to 2.8 m2/g for Cr,Ta-TiO2 and Rh-SrTiO3, respectively. Both Cr,Ta-TiO2 and Rh-SrTiO3 particles were connected with each other, i.e., “necking growth” was
![]()
Figure 5. STEM images of Pt/Cr,Ta-TiO2 (a) and Ru/Rh-SrTiO3 (b). Arrows show cocatalysts, either Pt or Ru.
observed due to rather high calcination temperature and long calcination time (900˚C for 24 h and 1000˚C for 10 h). Nanometer order of Pt on Cr,Ta-TiO2 and Ru on Rh-SrTiO3 could be observed (indicated by an arrow), and Pt sizes were larger than Ru the reason for which is not clear. From these observations, we also confirmed the deposition of Pt and Ru cocatalysts on Cr,Ta-TiO2 and Rh-SrTiO3, respectively.
3.2. Half Reactions of Water-Splitting
We examined the evolution of H2 and O2 by Ru/Rh-SrTiO3 and Pt/Cr,Ta-TiO2 in the presence of I− and 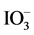 as sacrificial agents, respectively, under irradiation with visible light (420-nm LED) as shown in Figure 6. H2 evolution proceeded over Ru/Rh-SrTiO3 in the presence of I−. According to our previous paper [19] , only the negligibly small amount of H2 was observed over bare Rh-SrTiO3 in the presence of I−. Thus, we concluded that in the case of Rh-SrTiO3, the cocatalyst Ru on Rh-SrTiO3 was considered as the active site for H2 evolution. O2 evolution was detected for Pt/Cr,Ta-TiO2 (Pt/Ti0.986Cr0.007Ta0.007O2) in the presence of
as sacrificial agents, respectively, under irradiation with visible light (420-nm LED) as shown in Figure 6. H2 evolution proceeded over Ru/Rh-SrTiO3 in the presence of I−. According to our previous paper [19] , only the negligibly small amount of H2 was observed over bare Rh-SrTiO3 in the presence of I−. Thus, we concluded that in the case of Rh-SrTiO3, the cocatalyst Ru on Rh-SrTiO3 was considered as the active site for H2 evolution. O2 evolution was detected for Pt/Cr,Ta-TiO2 (Pt/Ti0.986Cr0.007Ta0.007O2) in the presence of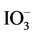 . Figure 6 also includes the O2 evolution in the presence of Pt/Ti0.982Cr0.009Ta0.009O2 quoted from our paper [20] . We could enhance the O2 evolution by changing the amounts of Cr and Ta at Ti. The present Cr,Ta-TiO2 (Ti0.986Cr0.007Ta0.007O2) is the optimized composition for H2 evolution for now.
. Figure 6 also includes the O2 evolution in the presence of Pt/Ti0.982Cr0.009Ta0.009O2 quoted from our paper [20] . We could enhance the O2 evolution by changing the amounts of Cr and Ta at Ti. The present Cr,Ta-TiO2 (Ti0.986Cr0.007Ta0.007O2) is the optimized composition for H2 evolution for now.
3.3. Two-Step Overall Water Splitting
We next examined water-splitting by the Ru/Rh-SrTiO3 and Pt/Cr,Ta-TiO2 photocatalysts under visible-light irradiation (420-nm LED) for ~350 h in the presence of 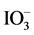 (/I−) as a redox mediator (Figure 7). As shown in Figure 7 in the first cycle, H2 and O2 evolutions were initially supressed, but then increased. In contrast, in the second and third cycles, such an induction period for H2 and O2 evolutions was not observed. Although the reason for the delay in the evolutions was unclear, such phenomenon is frequently encountered. It may be due to the surface restructuring of the photocatalysts and/or cocatalysts, or due to the consumptions of holes and electrons for the residual contaminants on the surfaces of the photocatalysts and remaining oxygen in aqueous solution, respectively. In the third cycle, water-splitting activity decreased likely due to the
(/I−) as a redox mediator (Figure 7). As shown in Figure 7 in the first cycle, H2 and O2 evolutions were initially supressed, but then increased. In contrast, in the second and third cycles, such an induction period for H2 and O2 evolutions was not observed. Although the reason for the delay in the evolutions was unclear, such phenomenon is frequently encountered. It may be due to the surface restructuring of the photocatalysts and/or cocatalysts, or due to the consumptions of holes and electrons for the residual contaminants on the surfaces of the photocatalysts and remaining oxygen in aqueous solution, respectively. In the third cycle, water-splitting activity decreased likely due to the
![]()
Figure 6. Time courses of H2 and O2 evolution resulting from the half reaction of waterbyRu/Rh-SrTiO3 and Pt/Cr,Ta-TiO2 photocatalysts irradiated with visible light (420-nm LED) in the presence of I− and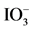 , respectively. O2 evolution was also included over Pt/Ti0.982Cr0.009Ta0.009O2 irradiated with 420- nm LED light in the presence of
, respectively. O2 evolution was also included over Pt/Ti0.982Cr0.009Ta0.009O2 irradiated with 420- nm LED light in the presence of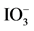 . The data were replotted from ref. [20] .
. The data were replotted from ref. [20] .
![]()
Figure 7. Time courses of H2 and O2 evolution resulting from photocatalytic overall water-splitting by Ru/Rh-SrTiO3 and Pt/Cr,Ta-TiO2 photocatalysts under irradiation with visible light (420-nm LED). The reaction was allowed to proceed for ~350 h with twice evacuations of the system. Those by Pt/Ti0.96Cr0.02Ta0.02O2 and Pt/Ti0.982Cr0.009Ta0.009O2 under 420-nm LED light were also included in the 3rd cycle. The data were replotted from ref. [20] .
detachment of cocatalysts. In addition, the amount of water decreased and the partial sample powders attached to the inner wall of the reaction vessel as the repeating cycle increased. These are possible reasons for the decrement in the water-splitting activity in the third cycle. However, H2 to O2 were evolved in nearly stoichio-metric (~2 to 1) amounts during each irradiation cycle.
It is well-known that the electron-and-hole transfers between the redox medi- ators needed for the two-step system and two types of photocatalysts are not efficient. In addition, the redox mediators usually give undesirable effects, e.g., backward reactions to form water from evolved H2 and O2. However, the H2 and O2 generation rates form the overall water-splitting reaction (0.17 and 0.09 µmol h-1, respectively, in the second cycle) were more than one-half of those from the half reaction of water-splitting (0.29 and 0.16 µmol∙h−1 for H2 and O2 rates, re- spectively, in Figure 6). To our knowledge, the efficiency of H2 and O2 gene- rations from the overall water-splitting is rather high. The reason is still unclear; however an affinity between photocatalysts and the redox mediators in the con- structed system would be suitable because it actually limits those combinations to several systems [13] .
As determined by XRF analysis, the Ru/Rh-SrTiO3 sample contained Ru at a molar percentage of 1 ´ 10−2 (1 mol%). Therefore, because a total of 10 mg of Ru/Rh-SrTiO3 was used in the water-splitting experiment as the H2-photo- catalyst, the amount of Ru was 5 ´ 10−1 µmol. As the total amount of H2 generated during the 350-h experiment was 53 µmol, we estimated that the turnover number, the ratio of total amount of produced to H2 to that of Ru co-catalyst, was ~100, which exceeded 1, indicating that this reaction proceeded catalytically. These results demonstrate that the photocatalytic overall water- splitting was achieved in this system. The possible mechanism for the overall water splitting by Pt/Cr,Ta-TiO2 and Ru/Rh-SrTiO3 is schematically illustrated in Figure 8. Visible light-excited electrons in the conduction band of Rh-SrTiO3 are thought to reduce H2O and generate H2 and holes in the valence band of Cr,Ta-TiO2 oxidize H2O and produce O2. In contrast, visible-light-excited holes in the VB of Rh-SrTiO3 and electrons in the isolated Cr 3d state contribute to the turnover of I− to , respectively, and vice versa [19] [20] .
, respectively, and vice versa [19] [20] .
When we compare our previous system (Pt/Ti0.96Cr0.02Ta0.02O2 and
Pt/Ti0.982Cr0.009Ta0.009O2 [20] ) as indicated in the third cycle in Figure 7, the H2 and O2 generation rates acheved ~100 times higher in the present system, constructed by Ru/Rh-SrTiO3 and Pt/Cr,Ta-TiO2. It is plausible that the com- position of Cr,Ta-TiO2 (Ti0.986Cr0.007Ta0.007O2) was optimized as the O2- photo- catalyst and that the effective Ru/Rh-SrTiO3 was utilized as the H2- photo- catalyst. In addition, the commercial P-25 TiO2 (Degussa) and famous nitrogen- doped TiO2 could evolve only a trace amount of H2 under the identical 420-nm LED (data not shown) and could not achieve the overall water-splitting. So the present system would be one of the candidates for obtaining H2 as it consists of environmental-friendly elements.
4. Conclusions
We established a two-step overall water-splitting system that is sensitive to visible light by utilizing TiO2-based and SrTiO3-based photocatalysts that simulta-
![]()
Figure 8. Schematic illustrations of spontaneous H2 and O2 evolution by Pt/Cr,Ta-TiO2 and Ru/Rh-SrTiO3 under irradiation with visible light in the presence of  /I− redox mediator.
/I− redox mediator.
neously evolve H2 and O2 at a molar ratio of ~2 to 1 efficiently in the presence of ![]() (/I−). However, it is necessary to further enhance the visible light sensitivity. This will require the preparation of these TiO2- and SrTiO3-based photocatalysts which are deficient-free, more highly crystallized, and smaller-sized particles. As is known, TiO2-based photocatalysts are attractive. So, we are now optimizing the types of dopants and concentrations to further enhance the visible-light sensitivity of TiO2-based photocatalysts. Moreover, we have recently developed a solid-state two-step overall water-splitting photocatalyst, in which the H2- and O2-evolution photocatalysts were connected via silver. In this system, distilled water could be split without a redox mediator. The construction of solid-state systems using TiO2-based and SrTiO3-based photocatalysts in the present study may be advantageous for industrial and practical applications, as no chemicals are required as redox mediators.
(/I−). However, it is necessary to further enhance the visible light sensitivity. This will require the preparation of these TiO2- and SrTiO3-based photocatalysts which are deficient-free, more highly crystallized, and smaller-sized particles. As is known, TiO2-based photocatalysts are attractive. So, we are now optimizing the types of dopants and concentrations to further enhance the visible-light sensitivity of TiO2-based photocatalysts. Moreover, we have recently developed a solid-state two-step overall water-splitting photocatalyst, in which the H2- and O2-evolution photocatalysts were connected via silver. In this system, distilled water could be split without a redox mediator. The construction of solid-state systems using TiO2-based and SrTiO3-based photocatalysts in the present study may be advantageous for industrial and practical applications, as no chemicals are required as redox mediators.