Heterogeneous Microstructure and Distribution of Trace Elements in Coral Stylophora pistillata Nursed in the Phosphate Loading Berth Site in the Gulf of Aqaba ()
1. Introduction
Terrestrial runoff into seawater leads to increasing loads of nutrients, sediments and pollutants discharged from the land like using and handling of fertilizers that contain phosphate [1] [2] , which is considered to be a growing concern for most of the countries endowed with coral reefs [3] [4] . This is referred to high sensitivity of coral reef ecosystems to changes in the surrounding physicochemical environment [5] .
Seawater contains trace elements in the form of K+, Na+, Mg2+, Ca2+, Cl− and 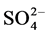 which have significant impacts on the formation and elemental composition of marine skeletons like corals [6] . Trace elements are secreted within the coral skeleton through either direct replacement of calcium (or carbonate) in coral aragonite structure for divalent ions [7] [8] [9] , while non-divalent ions are trapped by the inclusion of detritus materials into skeletal pore spaces, uptake of organic materials, incorporation of metals into coral skeletons, or coral feeding [7] [8] [10] [11] [12] . The incorporation of trace elements in coral skeletal aragonite crystals provides chronological proxies for physiochemical changes in the surrounding environment [12] [13] [14] .
which have significant impacts on the formation and elemental composition of marine skeletons like corals [6] . Trace elements are secreted within the coral skeleton through either direct replacement of calcium (or carbonate) in coral aragonite structure for divalent ions [7] [8] [9] , while non-divalent ions are trapped by the inclusion of detritus materials into skeletal pore spaces, uptake of organic materials, incorporation of metals into coral skeletons, or coral feeding [7] [8] [10] [11] [12] . The incorporation of trace elements in coral skeletal aragonite crystals provides chronological proxies for physiochemical changes in the surrounding environment [12] [13] [14] .
Conventional loading systems of phosphates produced as a constituent of fertilizer result in very high levels of dust pollution in the surrounding coastal area, diffused and transported along the sea by winds [15] . Phosphate is an extremely dry and dusty material and its solubility in seawater is relatively high (20 - 56 micrograms per liter, depending on particle size) [16] [17] . In Aqaba seawater, phosphate dust was found to be soluble over the annual water temperature range and some algae may be able to solubilize particulate phosphate [18] [19] .
Phosphate pollution has also been implicated as a contributor to the decline of reef ecosystems in the Gulf of Aqaba [20] [21] [22] [23] . Phosphate was reported to cause 36% inhibition of calcification in Stylophora pistillata at 2 ppm concentrations in seawater, and stop it completely (99%) at 100 ppm [24] . It was found that phosphate is responsible for increasing the death rate of Stylophora pistillata coral [25] while other study reported tolerance of coral species Stylophora pistillata to phosphate [26] . Al- Sawalmih [27] in his studies on branches Stylophora pistillata that are nursed in the Phosphate Loading Berth (PLB) site, found very low calcium composition in the aragonitic skeleton, about 10% of it in the control samples. He also found that these microstructures of these branches suffered from alteration due to dominance of organic fibers in the skeleton.
The Stylophora pistillata is among the important common coral species in the Gulf of Aqaba as well as in the Red Sea and known to be the fastest of the scleractinian corals. It is also very suitable for research because it can also be sampled without causing major damage to the colony [28] [29] .
In the present study, we investigate the heterogeneity of mineral content and trace elements in Stylophora pistillata corals in the Phosphate Loading Berth (PLB) site using Back-Scatter electron images and EDX techniques for elemental analysis. We also show the heterogeneous structure of the phases with different Ca concentrations in the micrometer scale within the skeleton.
2. Materials and Methods
Small fragments of the model branching coral Stylophora pistillata were cultured in a mid-water floating nursery along the Jordanian coast of Gulf of Aqaba [30] . The fragments developed into small colonies to be used as bio-indicator for marine pollution in the selected sites.
Coral samples were collected from the nurseries in the Phosphate Loading Berth (PLB) site (Figure 1), which lies about 3 km south of Aqaba city and is the single port for loading and exporting phosphate ore in Jordan.
Coral samples were cut perpendicular to the skeletal growth using low speed diamond saw (BUEHLER Isomat, Germany) and cross section surfaces were further polished using ethyleneglycol lubricant to provide a flat surface that was coated with Au for the SEM imaging.
Field-Emission Scanning Electron Microscope (JEOL JSM-7500F) with an Oxford Instruments detector was used for performing the EDX analysis to measure the elemental content in the coral samples and their corresponding emission spectra, as well as acquiring Back-Scatter Electron (VSE) microscopy images for the cross section of the sample. EDX analysis usually involves the generation of an X-ray spectrum from the entire scan area of the SEM, which shows the counts of X-rays received and processed by the detector and the energy level of those counts. The EDX instrument software associates the energy level of the X-rays with the elements found in the scan area.
![]()
Figure 1. Map of the Gulf of Aqaba where the location of the Phosphate Loading Berth (PLB) is indicated.
3. Results and Discussion
Figure 2 shows a Back-Scattering Electron (BSE) image for a polished surface of the coral sample. BSE can be used for rapid discrimination of phases in multiphase samples with high contrast of electron density, like organic and mineral phases, based on less- and more Ca concentrations.
Back-Scattered Scanning Electron Microscopy (BS-SEM) images of coral samples collected from the Phosphate Loading Berth (PLB) in the Jordanian Gulf of Aqaba (Figure 2) showed also that coral fragments from the phosphate pollution site is heterogeneous in the micrometer scale comprised of less-mineralized parts with very low calcium concentrations and mineralized parts with relatively higher calcium concentrations.
In Figure 2, the dark regions resemble the less-Ca phases and the bright ones indicates for the mineralized areas. This heterogeneous structure comes in accordance to the last study by Al-Sawalmih [27] on Stylophora pistillata coral samples from the phosphate pollution site, reporting dominance of organic/low-mineralized microstructure in SEM images and elemental analysis. This can be attributed to the role of phosphate in enhancement of coral zooxanthellae photosynthesis that is responsible for synthesis of organic matrix in the skeleton [31] .
Four positions in the cross-section in the BSE image (Figure 2) were selected for elemental analysis using Energy-Dispersive X-rays (EDX); (1) and (2) in the less- mineralized “dark” region and (3) and (4) in the more mineralized “bright” region. The recorded EDX spectra from the positions 1-4 (Figure 3) were used by the instrument software to determine the weight percentage (wt%) of Oxygen (O), Carbon (C) and Calcium (Ca) in addition to the trace elements: N, Mg, S, Cl, Ni and Sr. The full quantitative elemental analysis for the positions 1-4 are presented in Table 1.
In all locations, Ca concentrations were very low as expected and recorded in previous study on such coral in the same site [27] which was only 2.56 wt%, which is around 12% of the average in the control sample (21.95 wt%) but with a focus area
![]()
Figure 2. Back-Scatter Electron image of coral cross section. The four positions were selected for the EDX elemental analysis. The scale bar is 2 micrometer.
![]()
Figure 3. EDX spectra of the four selected positions for elemental analysis.
![]()
Table 1. Concentrations in wt% of C, O, Ca, and trace elements in the EDX scanned positions 1-4 in the coral cross section BSE image (Figure 2).
averages over the range of 100 m. In our study, the focus area for the EDX analysis was in the range of 1 m in order to map the heterogeneity in trace element distribution within the skeleton microstructure.
In Figure 4, the spatial heterogeneity in C, O, and Ca concentrations are presented, showing in addition low Ca concentrations in the dark regions (1) and (2), higher C concentrations and lower O amounts. C percentage decreases with increase of mineral composition while O is decreasing. This can be also noticed in the elemental spectra in Figure 3. This is an obvious result; when organic material is dominating then the C concentrations becomes more. Quantitative correlation between concentrations of these
![]()
Figure 4. Bar chart representation of the concentrations in wt% of C, O, and Ca in the EDX scanned positions 1-4 in the coral cross section BSE image (Figure 2).
elements needs to be further investigated based on expanded experimental work and literature survey. In the less-mineral region, position (2) has quite higher Ca than (1) because the last is located close to the less- and higher mineralized interface (Figure 2), which may give an indicator to the gradient behavior of Ca concentration through this boundary.
The amount of trace elements (N, Mg, S, Cl, Ni, and Sr) in the four scanned positions in the coral sample cross section (Figure 2) are shown in Figure 5. The EDX elemental analysis shows that the coral samples contain considerable amounts of trace elements (see Figure 1) which is strongly related to the chemistry of surrounding seawater during skeleton formation [10] [32] [33] .
Except for Chlorine (Cl), trace elements in the position 1 of the lowest calcium content were very low (<0.1 wt%) so their amounts can’t be considered due to possible uncertainty in their values. Trace elements concentrations can be reliably considered only in regions of high Ca concentrations.
The relatively high Cl concentration in position (1) which is 3 times more than it in other positions cannot be explained. Chlorine (Cl) exist in coral skeletons (but not in the aragonitic structure), as potassium chloride or as sodium chloride phases and is considered to be toxic for corals [34] . The main source of excess of Cl in seawater is sewage treatment that during floods which may be mixed with seawater, in addition to biofouling treatment in industrial effluents [35] .
In Figure 5, Na and Sr are the elements of the highest concentrations among other trace elements, which is typical for coral skeletons where Na and Sr are higher than any other trace element [36] . Moreover, it can be well noted from Figure 5 that Na concentrations are positively very well correlated with Ca concentrations. The average Na/Ca ratio for all scanned positions is 0.072 ± 0.005. For Sr/Ca ratio, if we consider only the higher-mineralized part for accurate estimation, its averaged value become 0.062 ±
![]()
Figure 5. bar chart representation of the concentrations in wt% of Ca, and trace elements in the EDX scanned positions 1-4 in the coral cross section BSE image (Figure 2).
0.015. However, the method used for this study is not optimal for calculating trace- element/Ca (mmole/mole) ratios.
Although Na is not a divalent cation (monovalent ion), i.e. it does not satisfy the electrostatic valence requirements of the Ca position in the aragonite lattice, but being the second most abundant ion in seawater [37] , in addition to the similarity of its ionic radius, Na transfers rapidly from sea water into the crystallizing skeletal material [38] . The charge imbalances resulting from the incorporation of Na+ into aragonite lattices is compensated by lattice defects and distortions such as vacancy sites and interstitial ions that occur at higher density at the center of calcification [36] [39] .
Strontium (Sr) ion is a divalent cation as magnesium (Mg), which can be secreted within the coral skeleton through direct replacement of calcium (or carbonate) in coral aragonite structure [7] [8] [9] . Sr is preferentially incorporated into the organically precipitated aragonitic skeletal material although having greater ionic radius than Ca [40] . Sr levels in the coral aragonitic skeleton has long been recognized as an indicator of seawater temperature at the time of coral growth, but anthropogenic pollution in seawater may effect on the temperature record [41] .
Mg is generally found in much lower concentrations in aragonitic skeletons due to its smaller ionic radius than Ca [42] , although being the third most abundant ion in seawater, behind sodium (Na) and chloride (Cl) [6] .
Due to its inhibition of calcification, high levels of phosphate are inversely associated with decreasing concentrations of strontium in the corals [43] . Nevertheless, morphological alteration of the coral skeleton due increased levels of phosphate suggests also that strontium may provide an excellent indicator of environmental stress related to anthropogenic-induced elevated nutrient levels [41] .
Sulphur (S) was not detected in our sample. It is the forth abundant ion in seawater (as sulfate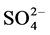 ) following close behind Mg [37] . It exists in coral skeletons as occupying specific sites in the crystal lattice of marine aragonites [44] [45] .
) following close behind Mg [37] . It exists in coral skeletons as occupying specific sites in the crystal lattice of marine aragonites [44] [45] .
Nickel (Ni) concentrations in the four scanned positions were almost not detected. It is weakly incorporated into the aragonite skeleton relative to calcium concentrations due to structural incompatibility [46] . Although Ni is vital for the function of many organisms, concentrations in some areas from both anthropogenic release and natural sources might increase its concentrations to toxic levels to living organisms [47] [48] [49] .
The low calcium in corals that are stressed by phosphate dust pollution, is due to the enrichment of phosphate as nutrient in seawater which cause inhibition of calcification in corals and coralline algae as well [25] [50] . It was reported that phosphate in seawater decrease calcification in the corals Pocillopora damicornis [51] , through increasing eutrophication which effects on survival and stability of reef communities by disrupting normal carbon cycle functions [52] [53] [54] . However, the mechanisms by which dissolved inorganic nutrients like phosphates can disturb corals are subject to controversial debate [55] .
The environmental impacts of the deposited phosphate dust on marine ecosystem include not only siltation of the coral reef and depression of coral growth (Hawkins et al., 1991) but also increasing suspended solids and water turbidity, reduction of water clarity and light penetration [56] . Phosphate dust also has physical effects in reducing the light intensity and increasing sedimentation, both of which have been shown to have deleterious effects on reefs [57] . Reduced light, excess phosphate and sedimentation were found to be responsible for reduced calcification and increased mortality of corals [25] .
4. Conclusion
Distribution of calcium in different very low concentrations was found in skeleton of corals that are exposed to phosphate dust and heterogeneous in the micrometer scale. This can be attributed to the well-known inhibition process of calcification by increase of phosphate concentrations at the time that the corals were formed. Dominating trace elements were only Na and Sr, which is typical for coral skeleton constituent. Chlorine was found to be higher in the lowest-calcium concentrations than other regions. Further elemental analysis and microstructure studies could be conducted on other coral species and several skeletal organisms that live in the Phosphate Terminal coastal site in order to find out their response to phosphate pollution. Moreover, trace element/Ca ratios should be considered in future similar studies using suitable investigation techniques for complete analysis.
Acknowledgements
The authors are indebted to Prof. Fuad Al-Horani for providing the coral samples used for this study and to Prof. Peter Fratzl for giving the chance to use the SEM laboratory at the Max-Planck Institute for Colloids and Interfaces (MPIKG). Many thanks to Susann Weichold and Heike Runge of the SEM lab Germany for their support and help during the measurements, also we are thankful to the divers Ali Al-Njadat and Omar Al-Momany for collecting the coral samples used in this study.