Optimizing Hydraulic and Chemical Properties of Iron and Aluminum Byproducts for Use in On-Farm Containment Structures for Phosphorus Removal ()
1. Introduction
Water quality impairment from phosphorus (P) enrichment is a major concern in many areas of the U.S., with agriculture purported to be a major contributor [1] [2] . Runoff from the land directly around broiler production facilities has been shown to be enriched with nutrients that could potentially contribute to nearby surface water quality impairment [3] [4] . Removing P from runoff water prior to it leaving the production area is more cost-effective than treating the receiving water once impaired [5] . While P can be removed during movement along a grassed waterway between poultry houses, P can accumulate in the soil, which can then represent a long-term source of P for off-site transport in runoff [6] . Removal of dissolved P from surface flow is more challenging than for particulate P and most remedial measures for dissolved P focus on removal of P as close to its source as possible [7] [8] . Therefore, conservation practices that trap P prior to the P leaving the production area are needed for protection of surface water quality.
Several P removal systems, primarily designed for municipal and construction site stormwater treatment, are commercially available [9] [10] . However, their use on the over 1600 privately owned poultry houses in the Illinois River watershed in northwest Arkansas and northeast Oklahoma is cost-prohibitive [11] . The effectiveness of the commercial systems is limited by their low hydraulic conductivity, which can restrict flow through the P-removal media during high-flow events. The coarser particle-size fraction used in the overflow weir design is adequate for hydraulic transmission, but results in reduced reaction time, which can limit P removal from solution [12] .
Materials specifically manufactured to adsorb P tend to be costly and are, therefore, impractical for use at a broiler production facility. However, industrial byproducts with high P-adsorption capacities do exist. Water treatment residuals (WT) are one such class of byproducts. They are produced in large quantities by municipal drinking water treatment plants when coagulants, such as alum, are added to raw water to flocculate solids suspended in the water. A local drinking water treatment plant in northwest Arkansas uses alum as a flocculent and the resulting “floc” is skimmed from the treated water and centrifuged to remove free water, then disposed of in landfills [13] .
Another locally-sourced product of potential use is an iron filter cake generated in the manufacturing process of steel belts for steel-belted, radial-ply tires. During the steel-belt production process, wastewater is created which contains ferrous iron, hydrochloric acid, and phosphoric acid. The pH of the wastewater is adjusted to greater than 8.5 with calcium hydroxide to create metal hydroxides, after which an ionic polymer is added to flocculate the metal hydroxides. Water is removed from the flocculent to create a red mud (RM) filter cake, which is disposed of in landfills.
Byproducts similar to RM and WT have been shown to be potentially useful in enhancing P removal in constructed wetlands [12] , as once dried, these byproducts have high P adsorption capacities [14] [15] . The objective of this study was to evaluate the P-adsorption and water transmission characteristics of WT and RM byproducts that may have potential use as P-adsorbing materials in on-farm P removal systems. It was hypothesized that the WT and RM byproducts would have large P-adsorption capacities. However, WT contains P due to its use as an adsorbent to remove P in the drinking-water filtration process, and RM contains P due to its use as a cleaner in the manufacturing process to produce steel belts. Additionally, it was hypothesized that larger particle-size fractions of each of the byproducts would have greater HC and lower P-sorbing capacities than smaller particle-size fractions.
2. Materials and Methods
2.1. Byproduct Selection and Particle Sizing
Red mud, iron filter cake was obtained from a local manufacturing company and water treatment (WT) residual was obtained from a water treatment plant in northwest Arkansas. The byproducts were loaded onto a tarp-lined flatbed trailer. The WT and RM were sieved to remove material >12.5 cm and were air-dried. As both P adsorption and hydraulic conductivity were expected to be affected by byproduct particle size, particle-size fractions were subsequently selected to contain both small particle sizes for maximum P adsorption and larger particle sizes to allow for sufficient hydraulic conductivity. Screen sizes with 6.35-mm (0.25 inch) and 12.7-mm (0.5 inch) openings were chosen based on availability of wire mesh to allow processing of large volumes of material. The byproducts shrink during the drying process, resulting in a mixture of particle sizes ranging up to the mesh size of the screen. Additional crushing was required to generate the ≤2-mm-sized material. Once dried, samples were sieved into particle-size fractions of ≤2-, ≤6-, and ≤12.5-mm. The particle-size distribution of RM and WT in the three size classes were determined by sequential dry sieving (Table 1).
Polycarbonate columns 25.4-cm long and 7.62 cm in diameter were constructed (Figure 1). Inlet and outlet holes were drilled 2 cm from the bottom and top of column, respectively. Rubber stoppers were glued to the bottom of each column. One end of a piece of 0.95-cm-diameter tubing was attached to the inlet connector and the other end to a funnel mounted on a stand at a height to create 5 cm of hydraulic head. Another section of 0.95-cm-diameter tubing was attached to the outlet connector and the end of the tubing directed into a collection container. An 18-L reservoir was used to supply flow, regulated with a hose clamp, to the funnel to maintain a constant head.
2.2. Chemical Analyses
Initial total N concentrations of RM and WT were determined by dry combustion (Nelson and Sommers, 1996). Initial total P (TP) and total K were determined by inductively coupled, argon-plasma, optical emission spectroscopy following nitric acid
![]()
Table 1. Particle-size distribution (by volume) of red mud (RM) iron filter cake and water treatment (WT) residual byproduct materials.
![]()
Figure 1. Column design for phosphorus adsorption column study, also used for hydraulic conductivity determinations (adapted from [16] ).
digestion (PerkinElmer Optima 7300, Waltham, MA).
Phosphorus adsorption properties of ≤2 mm-sized RM and WT were determined by batch equilibrations, where 1 g of air dried byproduct was shaken end-over-end (60 rpm) with 40-mL distilled deionized water containing P of varying concentrations (0 to 1000 mg∙L−1 as K2PO4) for 60 min at 25˚C. After shaking, samples were centrifuged for 10 minutes at 5250 x g and 25˚C, decanted, and filtered through a 0.45-µm membrane filter. Dissolved reactive phosphate (DRP) concentrations of filtrates were determined by the colorimetric molybdenum-blue method of Murphy and Riley [17] . Equilibrations were conducted in triplicate and adsorbed P calculated as the amount of P added minus the amount remaining in solution after shaking. Phosphorus adsorption isotherms were constructed by plotting the equilibrium solution P concentration (C) and P adsorbed per unit weight of byproduct (x/m). Phosphorus adsorption maxima (Pmax) and binding energy (B) of RM and WT were calculated used the Langmuir isotherm equation [Equation (1)] [18] :
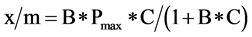 (1)
(1)
2.3. Hydraulic Properties
Hydraulic conductivities (HC) of the byproducts were determined for each of the three particle-size fractions using a modification of the constant-head method to determine HC of saturated soils [16] . This technique was designed to estimate P release from RM and WT residuals (Figure 1) [19] , but was modified to be used to also measure the HC of the two materials. The materials in each column were initially saturated with water until 5 mL were collected from the outlet of each column. Following initial saturation, a 5-cm constant head of pressure was used to input distilled water via the inlet at the bottom of the column. This method was chosen because, once dried, the RM and WT byproducts were hydrophobic and did not slake when wetted, making it difficult to remove air pockets and fully saturate the material when saturated from above, thus saturation from below was necessary.
Early attempts to saturate material from the bottom of the column and then apply a constant head from the top of the column were unsuccessful due to blockage by air pockets. Glass wool was then placed in the bottom of the column covering the inlet. A rubber ring and a wire screen were placed on top of the glass wool to hold the glass wool in place and create a level bottom, on top of which was placed 8 cm of byproduct. The alternative method allowed for improved flow and for a more consistent measurement of material HC, which closely represented field conditions. Red mud and WT were packed in columns to a constant bulk density (Table 2). Another layer of glass wool, sandwiched between two wire screens, was placed on top of the byproduct, below the outlet, to eliminate particle floating.
Hydraulic conductivity determinations were performed in triplicate on the three particle-size fractions of the RM and WT byproducts with water flow for 10 min. A constant head pressure was maintained using an elevated 18-L reservoir of water. Constant head hydraulic conductivity was calculated as:
 (2)
(2)
where HC is the saturated hydraulic conductivity (cm∙min−1), V is the volume of flow
![]()
Table 2. Bulk density of different particle-size fractions of iron (RM) and aluminum (WT) byproduct materials.
for a 10-min run (cm3), L is length of byproduct in column (cm), A is the internal area of column (cm2), t is the time of run (min), and H is the hydraulic head determined by H2-H1 from Figure 1 (cm).
2.4. P Adsorption Properties
The same particle-size fractions (≤2, ≤6, and ≤12.5 mm) of RM and WT used in the HC determinations were used in a P adsorption column study. This experiment was designed to simulate field conditions, where a P-containing solution flows through the byproduct. Phosphorus adsorption in this system was expected to be lower than that observed in traditional batch equilibrations, which had a longer contact time between P in solution and P-sorbing WT or RM materials (i.e., 60 and 10 min, respectively). The 18-L reservoir supplying the constant-head flow to the columns had a concentration of 6 mg∙P∙L−1, which was based on the average flow-adjusted runoff TP concentration from simulated rainfall plots adjacent to poultry house fans. Treatments were conducted in triplicate on the three particle-size fractions of both RM and WT byproducts. Outflow solutions were subsampled for analysis at the beginning of flow and every two minutes thereafter for a total of 30 min. Samples were filtered through a 0.45-µm membrane filter and DRP concentration was determined by the colorimetric molybdenum-blue method of Murphy and Riley [17] .
2.5. Statistical Analysis
A randomized block design with three replications for each P concentration tested was used to evaluate P adsorption of each byproduct at different solution-P concentrations. A one-factor analysis of variance (ANOVA) was conducted on results from the batch equilibration study for each byproduct. A homogeneity of variance test was conducted to compare HC of the ≤6- and ≤12.5-mm particle-size fractions of RM and WT. A homogeneity of variance test was also conducted to compare P removal of the ≤2-, ≤6-, and ≤12.5-mm particle-size fractions of RM and WT. Since variances were not homogenous for either HC or P removal, which violated the requirements for a two-factor ANOVA separate one-factor ANOVAs were conducted to evaluate the effect of byproduct on HC and effect of particle size on HC and to evaluate the effect of byproduct on P-removal capacity and effect of particle-size fraction on P-removal capacity. Significance was judged at the p < 0.05 level unless noted otherwise. Wolfram Mathematica (online beta version 2014, Wolfram, Champaign, IL) was used to conduct all statistical analyses.
3. Results and Discussion
3.1. Byproduct Composition
The initial TP concentration of the RM and WT was 33.9 and 1.2 mg∙TP∙g−1, respectively (Table 3). However, initial P concentrations were not a concern due to the fact that P adsorbed by these residuals is not released unless the material is acidified (i.e., dissolution of Fe and Al compounds) or becomes anaerobic (i.e., reductive dissolution of Fe compounds) [12] [14] . Initial N and K concentrations in the RM were below detection limits of 1.0 mg∙kg−1 for N and 100 mg∙kg−1 for K. The WT initially contained 7700 mg∙total∙N∙kg−1 and 1400 mg∙K∙kg−1. Both byproducts had a pH of 7.0 (Table 3).
3.2. Isotherms
Phosphorus adsorption determined by batch equilibrations reflects the remaining capacity of RM and WT to adsorb further P. Adsorption capacities between replicates did not differ (p > 0.05; Table 4). Over 90% of added P was removed by RM and WT, up to P applications of 13 and 3 mg∙P∙g−1 of byproduct, respectively (Table 4). The linearized form of the Langmuir equation was used to calculate P adsorption maxima and binding energies (see Equation (1)) (Figure 2):
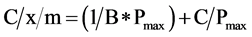 (3)
(3)
A plot of C/x/m against C should be a straight line if the Langmuir equation describes the isotherm, and the slope of the line is equal to 1/Pmax. As shown in Figure 3, the Langmuir equation described these isotherms (R2 > 0.96). The calculated Pmax was 25 and 10 mg∙P∙g−1 for RM and WT, respectively. Table 5 summarizes previously reported Pmax values for several P-sorbing materials, which range from 0.0003 mg∙P∙g−1 for limestone to 31.97 mg∙P∙g−1 for West Virginia ochre, an iron mining byproduct. The Pmax value for RM was greater than 87% of the materials listed and that for WT was greater than 74% of those listed. Clearly, the locally sourced RM and WT byproducts retain a large capacity to adsorb further P from solution.
![]()
Table 3. Initial nutrient composition and pH of red mud (RM) iron filter cake and water treatment residual (WT) materials.
![]()
Table 4. Phosphorus removed from solution during batch equilibration of the ≤2-mm particle size red mud (RM) and water treatment (WT) residual materials.
*p-value result of comparison across replicates for each byproduct.
![]()
Figure 2. Description of the isotherms by the linearized Langmuir equation.
![]()
Figure 3. Phosphorus adsorption isotherms for ≤2-mm particle-sized byproducts.
3.3. Hydraulic Conductivity
Variability in HC for all byproduct particle-size fractions was observed between replicates as a result of particle settling in the columns. The HC was greater in 60% of the runs in the first 10-min replicate than the subsequent two 10-min replicates for all particle sizes of RM. The HC replications varied by 3.4, 3.1, and 10.5 cm∙min−1 for RM particle-size fraction treatments of ≤2-, ≤6-, and ≤12.5-mm, respectively. The HC was greater in the first 10-min replicate than the last two 10-minute replicates in 100% of the runs for all particle sizes of WT. The HC replications varied by 0.6, 3.2, and 56.6 cm∙min−1 for WT particle-size fraction treatments of ≤2-, ≤6-, and ≤12.5-mm, respectively. Due to the variability in HC for all byproduct treatments, the first 10-minute replications were treated as conditioning runs to allow for byproduct settling and were excluded from the final calculations of HC for RM and WT.
Hydraulic conductivity varied notably for both the RM and WT ≤12.5-mm particle-size fraction treatments, ranging from 8.3 to 25.7 cm∙min−1 for RM and from 15.7 to 75.0 cm∙min−1 for WT (Table 6). The greater variability in HC with larger byproduct particle sizes was partially attributed to variability in capillary flow due to finer particles settling and clogging flow pathways, which was observed when material was removed from the columns following completion of the study. The ≤2-mm particle-size fraction treatment for both byproducts showed visibly dry material in the interior of the column following the study, indicating that they did not fully saturate in the column, thus were excluded from further analysis. This was of limited concern as the ≤2 mm material is too fine to be of value in the field by itself as a P-adsorbing residual receiving large volumes of water.
Comparison of HCs for the ≤6- and ≤12.5-mm particle-size fraction treatments yielded no difference (p > 0.05) in HC between ≤6- and ≤12.5-mm RM (Table 6). How- ever, there was a significant difference (p = 0.02) in HC for the WT, with the ≤12.5-mm
![]()
Table 5. Maximum phosphorus (P) adsorption capacities of a variety of materials that have been considered as P adsorbents.
material five-fold greater than that for the ≤6-mm material (Table 6). Further, the HC of RM and WT was similar (p > 0.05) for both size fractions.
![]()
Table 6. Average and variance of hydraulic conductivity of red mud (RM) iron filter cake and water treatment (WT) residual as a function of byproduct particle-size fraction.
*p-value result of comparison across replicates for each byproduct.
The HC of the ≤6- ≤12.5-mm RM and WT materials all exceeded the capacity to transmit rainfall during a 25-yr, 30-min storm (6.6 cm, 0.22 cm∙min−1) [20] . For the ≤6 mm WT material, HC was greater than 5.5 cm∙min−1 in all but one replicate. These results suggest that both RM and WT material of ≤6-mm and ≤12.5-mm size fractions could accommodate water flow from a 25-yr, 30-min rainfall event.
3.4. P Adsorption Column Study
Following HC measurement, the columns were used to estimate P adsorption, although applying a constant-head flow simulated extreme high flow conditions and resulted in reduced contact time between P solution and byproduct. The amount of solution that passed through the columns during the 30-min sampling period varied with particle size. For the ≤2-mm material, half the solution volume was applied compared to ≤6-mm material, allowing for additional contact time between solution P and byproduct particles. The larger reactive surface area of ≤2-mm-sized material compared to other material sizes resulted in 98% P removal by RM and 94% P removal for WT byproduct (Table 7).
While total flow volume for the ≤12.5-mm RM and WT material was greater than for ≤6-mm material, there was significantly less surface area and less added-P sorbed by RM (30% of added P) and WT (11% of added P) (Table 7). Phosphorus removal by the ≤2-, ≤6-, and ≤12.5-mm RM and WT differed (p < 0.001; Table 7). The ≤2-mm RM combination removed 42% more P than the ≤6-mm material, which removed 28% more P than the RM ≤12.5-mm material. While P removed by the finest material was slightly greater for RM and WT (4%, p < 0.001), there was no difference in the amount of P removed between RM and WT ≤6-mm materials (Table 6). Removed P differed (p < 0.05) between RM and WT ≤12.5-mm particle-size treatment, with RM removing 18% more P than WT (Table 7). Combining the HC and P-adsorption results, it was suggested that because the ≤6-mm RM and WT treatments could accommodate water from a 25-yr, 30-min storm and adsorbed greater than 56% of the added P, reducing
![]()
Table 7. Average and variance of phosphorus (P) removal of red mud (RM) iron filter cake and water treatment (WT) residual as a function of byproduct particle-size fraction.
*p-value result of comparison across replicates for each byproduct.
the P concentration on average from 6 to 2.5 mg∙P∙L−1, this material was preferred to the ≤12.5-mm byproduct material for in-field use.
4. Summary and Conclusions
The ≤2-mm/RM and WT byproducts had a large capacity to adsorb additional P with Pmax values of 25 and 10 mg∙P∙g−1, respectively. The Pmax values for RM and WT were compared with other P adsorbents that have been evaluated, and the Pmax value for RM was greater than 87% of the materials evaluated, and that for WT was greater than 74% of those evaluated. The Pmax value of any byproduct used is important in determining the viability of a product to be used in terms of capacity and lifespan of on-farm P-removal systems. Further, the HC of both the ≤6- and ≤12.5-mm RM and WT materials were sufficient to transmit runoff resulting from a 25-yr, 30-min storm event.
The P adsorption column study allowed testing of the three particle-size fraction treatments of each byproduct under conditions that were more reflective of field situations around broiler houses than the batch equilibrium method used for the isotherm analyses. The ≤2- and ≤6-mm particle-size fraction treatments removed greater than 50% of P in solution, indicating viability for use in on-farm P-removal systems. Successful design and operation of an on-farm P-removal system utilizing the RM and WT byproducts would be dependent on both the HC and P-removal potential of the selected particle-size fraction. Ultimately, field testing of any design would be necessary to properly evaluate and ensure effectiveness. The optimal particle size from this assessment of RM and WT materials was the ≤6-mm particle-size fraction, which allowed for a combination of sufficient through flow of rainfall generated runoff, while retaining a capacity to adsorb at least 50% of added solution P.
However, the practical implications of this research are that use of these two locally- sourced byproduct materials contained in trays beneath broiler production house ventilation fans, has the potential to trap P exhausted from the fans. Further, as these materials are currently landfilled, their on-farm use could provide a potential cost saving alternative. Finally, the byproduct material, once saturated with P, could become of potential use as a long-term, slow-release fertilizer P source to area pastures.
Acknowledgements
This research was supported by a grant from the USGS 104B program funding.