1. Introduction
Iron and its alloys are widely used in many applications, which have resulted in research into the corrosion resistance in various aggressive environments [1] . Hydrochloric acid is generally used as “pickling acid” for steel. Its function is to remove undesirable oxide coatings and corrosion products. Corrosion inhibitors used in acid treatment solutions significantly reduce the overall and local pickling attack and the hydrogen absorption of steel strips [2] [3] . Corrosion inhibition has also been conciliated for a variety of natural products as corrosion inhibitors like Asteraceae extract [4] , alkaloids extract [5] for mild steel dissolution in HCl solution were studied. Bamboo leaf extract [6] , olive leaves extract (Olea europaea) [7] , Murraya koenigii leaves extract [8] and Ochrosia oppositifolia [9] function as green corrosion inhibitor due to the presence of phytochemical compounds which incorporate effective corrosion inhibition groups such as hydroxyl [OH], carbonyl [CO], NH, aromatic ring by blocking the active surface sites to reduce the corrosion of steel in HCl solution. The development of corrosion inhibitors is based on organic compounds containing N, O, S atoms and multiple bonds in the molecules that facilitate adsorption on the metal surface [10] . The existing data show that organic inhibitors act by the adsorption on the metal surface and protective film formation [11] . The adsorption of organic inhibitors at the metal/solution interface takes place through the replacement of water molecules by organic inhibitors molecules [12] [13] . Plant extract is low-cost and environmental safe, so the main advantages of using plant extracts as corrosion inhibitor are economic and safe environment. Therefore, plants and natural product extracts have been posed to achieve the target of employing as cheap, environmentally acceptable, abundant source, readily available and effective molecules having very high inhibition efficiency and low or zero environmental impact [14] . The inhibition performance of plant extract is normally ascribed to the presence of complex organic species, including tannins, alkaloids and nitrogen bases, carbohydrates and proteins as well as hydrolysis products in their composition. These organic compounds usually contain polar functions with nitrogen, sulfur, or oxygen atoms and have triple or conjugated double bonds or aromatic rings in their molecular structure, which are the major adsorption centers [15] .
This work focuses on the inhibitory effect of Catharanthus roseus and Turmeric extracts as green inhibitors for mild steel in 1 M HCl solutions using different techniques.
2. Experimental
2.1. Materials and Solutions
2.1.1. Composition of material Samples
The chemical composition of mild steel sample is shown in Table 1.
2.1.2. Test Solutions
The solution of 1 M hydrochloric acid (Test solution) were prepared for each experiment using analytical grade of hydrochloric acid (37%) and diluted with distilled water from the standard 6 M HCl solution. The concentration range of inhibitor was 50 to 300 ppm.
2.2. Preparation and solubility of plant Extracts
2.2.1. Catharanthus roseus
Fresh aerial parts of Catharanthus roseus sample were crushed to make fine powder. The dried plant sample was grounded to give 250 gm which was extracted by using dichloromethane as solvent three times. Then filtered and evaporated to give dichloromethane extract and then concentrated to dryness which yielded weight 5 gm. After
that, take suitable weight and soluble it in distilled water according to the concentration range of inhibitors. Figure 1 illustrates the photograph of Catharanthus roseus and Figure 2 illustrates the photograph of Turmeric plant.
2.2.2. Turmeric
The uses parts were the bark and the rhizomes of curcum. The sample were purchased from the local market and ground into a fine powder to give 500 g of powdered materials which extracted separately by soaking at room temperature for six hours with methanol (5L), then the methanolic extract of the sample was concentrated to nearly dryness under reduced pressure by using the rotary evaporator at 45°C to achieve the crude methanolic extract.
2.3. Composition of Plant extracts
The elemental compositions of leaves and flowers of Catharanthus roseus [16] are sho- wn in Table 2 and the nutritional compositions of Turmeric [17] are shown in Table 3.
![]()
Table 1. Chemical composition (wt%) of the mild steel.
![]()
Table 2. Elemental compositions (dry weight basis mg/g) of leaves and flowers of catharanthus roseus.
![]()
Table 3. Nutritional compositions of Turmeric.
2.4. Electrochemical measurement
A three-electrode cell including a working electrode, an auxiliary electrode and a reference electrode was used for the electrochemical measurements. The working electrodes were made of mild steel sheets which were embedded in PVC holder using epoxy resin with a square surface of 1 cm2. The auxiliary electrode was a platinum foil, the reference electrode was a saturated calomel electrode (SCE) with a fine Luggin capillary tube positioned close to the working electrode surface in order to minimize ohmic potential drop (IR). Each specimen was successive abraded by using SiC emery papers up to 1200 grit size, washed with bidistilled water and degreased in acetone then dried between filter papers. The working electrode was immersed in the test solution at open circuit potential for 30 min before measurement until a steady state reached. All the measurements were done in solutions open to atmosphere under unstirred conditions. All potential values were reported versus PDE. Prior to each experiment, the electrode was treated as before. Tafel polarization curves were determined by polarizing to ± 250 mV with respect to the free corrosion potential (E vs. SCE) at a scan rate of 0.5 mV/s. Stern-Geary method [18] used for the determination of corrosion current is performed by extrapolation of anodic and cathodic Tafel lines to a point which gives log icorr and the corresponding corrosion potential (Ecorr) for inhibitor free acid and for each concentration of inhibitor. Then icorr was used for calculation of inhibition efficiency (%IE) and surface coverage (θ) as in equation (1):
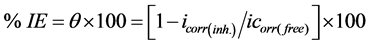 (1)
(1)
where icorr(free) and icorr(inh) are the corrosion current densities in the absence and presence of inhibitor, respectively.
Electrochemical impedance spectroscopy measurements EIS were carried out in a frequency range of 100 kHz to 100 mHz with amplitude of 5 mV peak-to-peak. . The experimental impedance was analyzed and interpreted based on the equivalent circuit. The main parameters deduced from the analysis of Nyquist diagram are the charge transfer resistance Rct (diameter of high-frequency loop) and the double layer capacity Cdl. The inhibition efficiencies and the surface coverage (θ) obtained from the impedance measurements are calculated from equation 2:
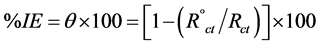 (2)
(2)
where 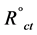 and Rct are the charge transfer resistance in the absence and presence of inhibitor, respectively
and Rct are the charge transfer resistance in the absence and presence of inhibitor, respectively
2.5. Hydrogen evolution Measurement (Gasometric Experiments)
The gasometric set up is essentially an apparatus that measures the volume of gas evolution from a reaction system. A two-necked flask was connected via a delivery tube to a burette which was in turn connected to a reservoir of paraffin oil. Test solution (100 ml) was then introduced into the reaction mild steel test sheets were dropped into the test solution and the reaction vessel immediately closed. The volume of hydrogen gas evolved by the corrosion reaction was estimated by the volume change in the level of the paraffin oil in the burette. The progress of the corrosion reaction was monitored by careful volumetric measurement of the evolved hydrogen gas at fixed time intervals. Experiments were conducted at 25˚C.
The inhibition efficiency IE is calculated by:
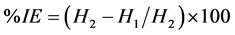 (3)
(3)
where H1 and H2 are the volume of hydrogen evolved in the presence and absence of inhibitor, respectively.
2.6. Scanning Electron Microscopy (SEM)
The mild steel specimens were immersed for 12 h in 1M HCl solution containing optimum concentrations (300 ppm CDE) of inhibitor. After 12 h, the specimens were taken out and dried. Examination of mild steel surface after 12 h exposure to 1 M HCl solution without and with inhibitor was carried by using (JEOL JSM-5500) scanning electron microscope.
3. Results and Discussion
3.1. Potentiodynamic Polarization
Electrochemical corrosion testing methods are extremely useful for the determination of corrosion behavior of metals, alloys and coatings in corrosive environments. The technique analyzes the corrosion behavior quickly, and can accurately identify the corrosion rate.
Potentiodynamic polarization curves of mild steel in 1 M hydrochloric acid in the absence and presence of plant extracts at 25˚C are illustrated in Figures 3-4. The numerical values of the variation of corrosion current density (icorr), corrosion potential (Ecorr), Tafel slopes (βa and βc), degree of surface coverage (θ) and inhibition efficiency (%IE) with the concentrations of plant extracts are given in Tables 4-5.
This indicates that:
1) The cathodic and anodic curves obtained exhibit Tafel-type behavior. Addition of extract inhibitors increased both cathodic and anodic over voltages and caused mainly parallel displacement to the more negative and positive values, respectively. The parallel cathodic and anodic Tafel curves in Figures 3-4 shows that the hydrogen evolution is activation-controlled and both of the reduction and dissolution mechanisms are not affected by the presence of the inhibitor [19] . So, it could be conducted that these inhibitors are mixed-type for mild steel in 1M HCl medium, which may be adsorbed on the cathodic sites of mild steel and reduce the evolution of hydrogen. Moreover, the adsorption of these compounds on anodic sites through the lone pair of electrons will then reduce the anodic dissolution of mild steel [20] [21] .
2) The corrosion current density (icorr) decreases with increasing the concentration of plant extract, which indicates that the presence of these extracts retard the dissolution of mild steel in 1M HCl solution and the degree of inhibition depends on the concentration and type of the inhibitor present.
3) The investigated extracts appeared to act as mixed type inhibitors, as it is shown from Figures 3-4 where both cathodic and anodic polarization curves are influenced by the presence of the investigated extracts in the corrosive media. In addition, the cathodic and anodic Tafel lines were more or less parallel.
4) The slopes of anodic and cathodic Tafel line (βa, βc) were slightly changed with increasing the concentration of the investigated extracts. This is indicates that the hydrogen evolution and metal dissolution reactions were activation controlled and the addition of inhibitors did not modify the mechanism of these processes.
3.2. Electrochemical impedance spectroscopy Measurements (EIS)
Electrochemical impedance spectroscopy provides a new method to characterize the film coverage on the electrode, which is related to charge transfer resistance (Rct). The
![]()
Figure 3. Potentiodynamic polarization curves for corrosion of mild steel in 1 M HCl in the absence and presence of different concentrations of Catharanthus extract at 25˚C.
![]()
Figure 4. Potentiodynamic polarization curves for corrosion of mild steel in 1 M HCl in the absence and presence of different concentrations of Turmeric extract at 25˚C.
![]()
Table 4. Potentiodynamic data of mild steel in 1 M HCl in the presence of different concentra- tions of Catharanthus extract at 25˚C.
![]()
Table 5. Potentiodynamic data of mild steel in 1 M HCl in the presence of different concentra- tions of Turmeric extract at 25˚C.
interface capacitance can also be used to determine the film quality [22] [23] . It is known that the coverage of an organic compounds on the metal surface depends not only on the structure of the organic compound and the nature of the metal, but also on the experimental conditions such as immersion time and concentration of adsorbent [24] [25] .
The corrosion behavior of mild steel in 1 M HCl solution in the absence and presence of different concentrations of the investigated compounds was investigated by using the EIS technique at 25˚C. Figures 5-6 show the Nyquist plots for mild steel. The diagrams are characterized by one time constants consisting of large capacitive loop at high to medium frequency and inductive loop at low frequency. The capacitive loop at high frequencies represents the phenomenon associated with the double electric layer. It arises from the time constant of electrical double layer and the charge transfer in corrosion process [26] [27] . The large capacitive loop makes an angle with the real axis and its intersection gives a resistance of the solution (Rs) enclosed between the working electrode and the counter electrode [28] . The presence of inductive loop may be attributed to the relaxation process obtained by adsorption species onto the electrode surface.
![]()
Figure 5. Nyquist plots for mild steel in 1 M HCl at different concentrations of Catharanthus extract.
![]()
Figure 6. Nyquist plots for mild steel in 1 M HCl at different concentrations of Turmeric extract.
From the impedance data given in Tables 6-7, we conclude that:
・ Rct increases by increasing the concentration of extract, giving consequently a decrease in the corrosion rate.
・ The high Rct values, are generally associated with slower corroding system [29] [30] .
・ The existence of single semicircle showed that single charge transfer process occurred during dissolution of mild steel which unaffected by the presence of investigated compounds.
・ Cdl values decreases with increasing the extract concentration. This is due to the gradual replacement of water molecules in the double layer by the adsorbed extract molecules which form an adherent film on the metal surface and leads to decrease in the local dielectric constant of the metal solution interface [31] .
3.3. Hydrogen evolution Measurement (Gasometric Experiments)
The technique of hydrogen gas evolution is used to follow the corrosion behavior of mild steel in 1M HCl solutions containing different concentrations of tested extract inhibitor. The curves of Figures 7-8 represent the variation in the volume of H2 gas evolved on mild steel coupons with the immersion time, when immersed in different concentrations of inhibitor solutions. Inspection of these curves reveals that hydrogen gas evolution starts after the elapses of a certain time from immersion of the mild steel in the test solution. This time is identified as the incubation period which is the time needed by the acid to destruct the pre-immersion oxide film and start dissolution of mild steel [32] .
It is noteworthy to see that the incubation period, in absence and presence of the inhibitor, is within the first 15 minutes of immersion of the steel coupons in the solution, and increases with increasing the additives concentration. After the incubation period, the volume of the H2 gas evolved increases linearly with time due to the possible simultaneous destruction of the porous film and the continuous dissolution of mild steel according to the reaction:
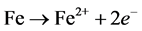 (R1)
(R1)
![]()
Table 6. EIS data of mild steel in 1M HCl in the presence of different concentrations of Cathar- anthus extract at 25˚C
![]()
Table 7. EIS data of mild steel in 1M HCl in the presence of different concentrations of Turmeric extract at 25˚C
![]()
Figure 7. Variation of the volume of H2 gas evolved on mild steel in 1M HCl solutions in absence and presence of different concentrations of Catharanthus extract, with the immersion time at 25˚C.
![]()
Figure 8. Variation of the volume of H2 gas evolved on mild steel in 1M HCl solutions in absence and presence of different concentrations of Turmeric extract, with the immersion time at 25˚C.
The accompanied iron dissolution process is the cathodic reaction to consume the electrons generated in the anodic process, as shown in Equation (2). In the acidic medium, the Hads, atomic hydrogen adsorbed on the metal surface reacts by combining with other adsorbed H atoms to form H2 gas, which bubbles from the surface according to:
![]() (R2)
(R2)
However it is noteworthy to see that the linear relation between the volumes of gas evolved V, in ml/cm2 changes with time t in minute, follows relation [32] .
![]() (4)
(4)
where r is the rate constant of the dissolution reaction.
Tables 8-9 represent the inhibition efficiency data from hydrogen evolution for mild steel in 1 M HCl solutions without and with various concentrations of plants extracts.
From all the results obtained from different techniques, may be compare between the values of inhibition efficiency for Catharanthus and Turmeric extracts (Table 10).
3.4. Scanning Electron Microscopy (SEM)
A photograph of the polished mild steel surface before immersion in 1M HCl solution is shown in Figure 9. The photograph shows the surface was smooth and without pits. A photograph of the mild steel surface after immersion in 1 M HCl solution (10 hrs.) is shown in Figure 10. The photograph revealed that, the surface was strongly damaged in the absence of plant extracts. A photograph of the mild steel surface after immer- sion in 1 M HCl solution (10 hrs.) containing 300 ppm of plant extract is shown in Figures 11-12. It was found that the faceting observed in figures disappeared and the surface
![]()
Table 8. Inhibition efficiency data obtained from hydrogen evolution technique for mild steel in 1 M HCl solutions without and with various concentrations of Catharanthus extract at 25˚C.
![]()
Table 9. Inhibition efficiency data obtained from hydrogen evolution technique for mild steel in 1 M HCl solutions without and with various concentrations of Turmeric extract at 25˚C.
![]()
Table 10. Inhibition efficiency data obtains from different techniques at 25˚C.
was free from pits and it was smooth. It can be concluded from Figures 10-12 that corrosion decreased largely in the presence of plant extract hence corrosion was inhibited strongly when plant extracts was present in the medium. The metallographical micrographs were exposed at magnification (X 1500). In the presence of 300 ppm of plant extracts, there is much less damage on the steel surface, which further confirm the inhibit ion action. Also, there is an adsorbed film adsorbed on mild steel surface.
In accordance, it might be concluded that the adsorption film can efficiently inhibits the corrosion of mild steel.
3.5. Mechanism of Inhibition Action of Catharanthus roseus and Turmeric Extract
3.5.1. Mechanism of Inhibition Action of Catharanthus roseus
The adsorption process is affected by the chemical structures of the inhibitors, the nature and charged surface of the metal and the distribution of charge over the whole inhibitor molecule. Chemical adsorption of Catharanthus extract arises from the donor acceptor interactions between free electron pairs of hetero atoms and p electrons of
multiple bonds as well as phenyl group and vacant d orbital's of iron [33] [34] . It has been reported that the adsorption of heterocyclic compounds occurs with the aromatic of heterocyclic compounds occurs with the aromatic rings sometimes parallel but mostly normal to the metal surface the inhibition action of Catharanthus extract does not occur by the simple blocking at the surface of mild steel, especially at high temperature. It has been studied that with the increase in temperature, the desorption effect of Catharanthus extract on mild steel surface decreased. Some of the hydrophilic groups with positively charged atoms (O+) desorbed from the surface of mild steel and did more work to prevent the H+ from getting nearer to the metal surface.
![]()
Figure 9. SEM micrographs of mild steel surface before immersion in 1 M HCl.
![]()
Figure 10. SEM micrographs of mild steel surface after 10 hrs of immersion in 1 M HCl.
![]()
Figure 11. SEM micrographs of mild steel surface after 10 hrs of immersion in 1 M HCl + 300 ppm of Catharanthus extract at 25˚C.
![]()
Figure 12. SEM micrographs of mild steel surface after 10 hrs of immersion in 1 M HCl + 300 ppm of Turmeric extract at 25˚C.
3.5.2. Mechanism of Inhibition Action of Turmeric Extract
As follows from potentiodynamic polarization and EIS measurements, corrosion of mild steel in 1 M HCl is retarded in the presence of Turmeric extract. The results clearly showed that the inhibition mechanism involves blocking of mild steel surface by inhibitor molecules via adsorption. In general, the phenomenon of adsorption is influenced by the nature of metal and chemical structure of inhibitor. Turmeric extract may act as a good corrosion inhibitor with at least one polar unit. These polar units can provide the free electron pairs and p-electrons, also hydroxyl group (?OH), which is an electron donator, moderately activated by virtue of presence of lone pair of electrons on the oxygen (O) atom. So turmeric extract can absorb into mild steel surface through the transference of free electron pairs and p-electrons to the d-orbital in iron atom, another previous studies show that the phosphate groups play an important role in the complexation process as a whole more effective donor that carboxylate groups. The presence of the aromatic ring increases the power complexing of these molecules. These studies present that a spectrophotometric method for the determination of complex is based on the chelation of the ligand with Fe (III) to formed complex. The IR spectroscopy revealed that iron ion coordinated through phosphonyl (P = O) and hydroxyl group (O-H) of the-hydroxyl phosphonate [35] .
4. Conclusions
On the basis of this study, the following conclusions can be drawn:
1) Catharanthus and Turmeric extracts act as inhibitors for mild steel corrosion in acidic medium.
2) Inhibition efficiency of Catharanthus and Turmeric extracts increases with increase in concentration of the inhibitor.
3) The values of inhibition efficiency indicate that Catharanthus extract is more effective than Turmeric extract.
4) The corrosion inhibition is probably due to the adsorption of the plant extracts on the metal surface and the blocking of its active sites by phenomenon of physical and chemical adsorption.
5) SEM reveals the formation of a smooth surface on mild steel in presence of Catharanthus and Turmeric extracts, which is probably due to the formation of an adsorptive film of electrostatic character.
6) Also the results indicate that Catharanthus and Turmeric extracts act as mixed type inhibitors.