Small Angle Neutron Scattering and X-Ray Diffraction Studies of Nanocrystalline Titanium Dioxide ()
1. Introduction
Microstructural aspects such as particle size and its distribution, defect structures of the materials having high technological applications are of great interest from both safety and performance perspectives. Nanophase oxide materials are interesting due to their potential and unique mechanical, chemical, electrical, optical and magnetic properties. Among various dioxides titanium dioxide (TiO2) has been suggested as being a material that could have a series of phases with hardness possibly approaching diamond [1,2]. Pure as well as doped nanostructured TiO2 materials are essential for the development of advanced electrochemical solar cells, photo catalytic, electron sensitive devices and optical coatings. TiO2 in its entire known three phases is technologically important material for numerous applications. TiO2 with rutile phase has been widely used as white pigment material because of its good scattering effect that protects materials from ultraviolet light. It has also been used for optical coatings, beam splitters because of its high dielectric constant and refractive index. The material also possesses good oil adsorption ability, tinting power, and chemical stability under strong acidic or basic conditions [3,4]. TiO2 with an anatase structure has been used as a photocatalyst for photodecomposition and solar energy conversion because of its high photoactivity [5-8]. TiO2 shows different electrical characteristics with oxygen partial pressure, because it has wide chemical stability and a non-stoichiometric phase region. For this reason, it can also be used as humidity sensor and high temperature oxygen sensors [9,10].
Nanocrystalline TiO2 powders are generally synthesized by different techniques such as the sulphate or chloride processes, sol-gel method, and hydrothermal synthesis [11-13]. All these synthesis techniques are vital and have advantages/disadvantages over each other but still there is a need for simple and cost effective process for the commercial production of TiO2 nano powders. For the practical applications of these high technological materials their physical and chemical properties are greatly dependent on the particle size. Therefore, it is necessary to know the true particle size, shape, and their distribution in these nanocrystalline materials. X-ray diffraction (XRD) is a powerful tool to identify the different phases of compound but small angle neutron scattering (SANS) is well suited technique for an analysis of microstructural characterization of such high technological materials, allowing for quantitative measurements of particle/pores morphology, particle size distribution. In this paper the results are demonstrated for the nanocrystalline commercial DEGUSSA P25 TiO2 for particle size determination and distribution by SANS and phase analysis by XRD. The results are being compared with earlier published data.
2. Experimental
The XRD pattern for TiO2 nanocrystalline powder was measured by Philips Xpert PRO diffractometer equipped with PW3015 generator, goniometer and X’celerator (SSD detectors) in the 2q ranges 20˚ - 90˚. The small angle neutron scattering measurement was carried out with the SANS instrument at the HANARO reactor of the Korea Atomic Energy Research Institute, Korea. The SANS instrument had a typical pinhole geometry using neutrons with wavelength of 5.08 Å. Wavelength spread was (Dl/l » 10%). The distance from sample to detector was 3 m. The measured q-value range was 0.1 - 1.8 nm–1. The scattering pattern of the sample was recorded with a two-dimensional position sensitive detector with an active area of 64.5 ´ 64.5 cm2, and were circularly averaged to produced one-dimensional intensity profiles. The scattering intensity data were reduced to absolute scale by using standard porous silica in 1 mm path length quartz cell. Scattering intensities were measured from TiO2 nanocrystalline powder filled in 25 mm diameter quartz cell with 1 mm path length.
3. Results & Discussion
Figure 1 shows the XRD pattern for the nanocrystalline TiO2 powder. All of the peaks on this pattern belong to rutile and anatase phases of this compound. These peaks have been marked as (A) for anatase and (R) for rutile phase respectively on the diffraction pattern. The general proportion of these phases in commercial TiO2 has been known as 75% for anatase and 25% for rutile [12]. In Figure 1, the XRD data gives this ratio as 75%:25% for anatase and rutile phases on the basis of maximum peak intensities ratios for the respective two phases. No traces of others TiO2 phases such as brookite or Zr based srilankite were observed which often appear in most of the nanocrystalline TiO2 powders prepared by different processes [12]. The particle size in the rutile phase was reported to be larger than that of anatase by different
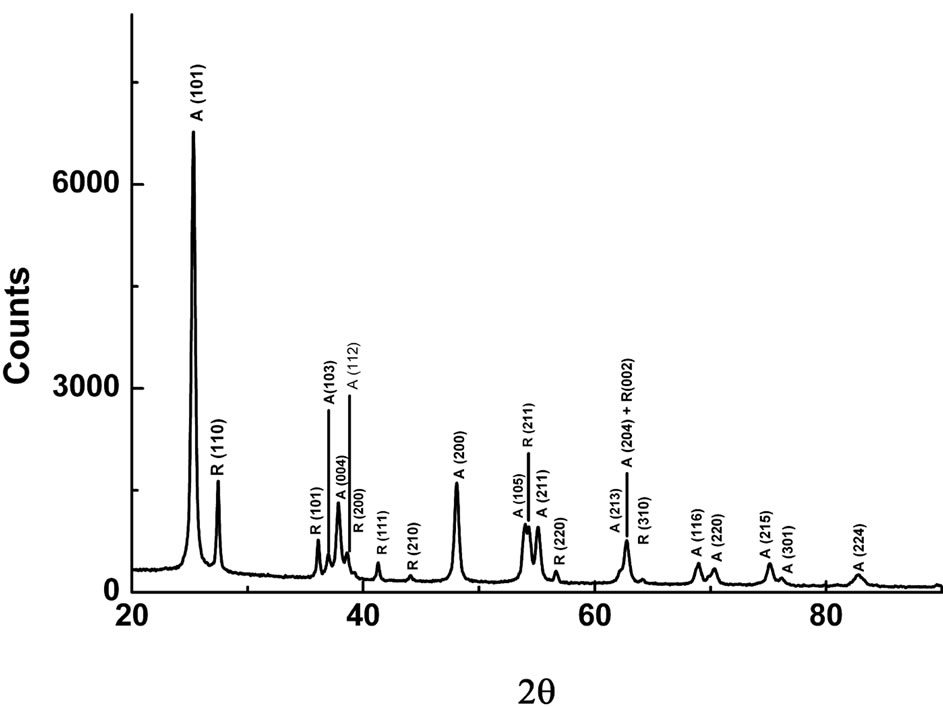
Figure 1. XRD pattern for the Degussa P25 nanocrystalline TiO2 powder.
synthesis processes because of the agglomeration process, which is the growth of primary TiO2 particles with an increase of thermal energy [12]. The above finding of the phases and their ratio in this study are well in agreement with the earlier published X-ray and synchrotron X-ray diffraction data on the same material [14,15].
Figure 2 shows the one-dimensional (1-D) SANS pattern for the TiO2 nanocrystalline powder circularly averaged from the 2-D measured pattern. The 2-D pattern (not shown here) clearly revealed isotropic scattered symmetric behavior, thus indicating that structures inside the powders are spherical. As the particles in the powder possess different sizes and internal structures, therefore the macroscopic differential scattering cross section for spherical particles as reported in [16] is given by;
 (1)
(1)
where  is the scattering contrast (
is the scattering contrast ( is the scattering length density) depending on the chemical composition of both the scattering centers and matrix, R is the radius of the spherical particles. N(R)dR is the number of the particles per unit volume with a typical size between R and R + dR. F2(Q, R) is the form factor which in the case of spherical particles is given by;
is the scattering length density) depending on the chemical composition of both the scattering centers and matrix, R is the radius of the spherical particles. N(R)dR is the number of the particles per unit volume with a typical size between R and R + dR. F2(Q, R) is the form factor which in the case of spherical particles is given by;
 (2)
(2)
The scattering length density was calculated with assumption that the particles smaller than 50 nm are dispersed equally in this material. The model fitting of the real space size distribution, log-normal size distribution to the scattering pattern was performed using non linear least square fitting [17];
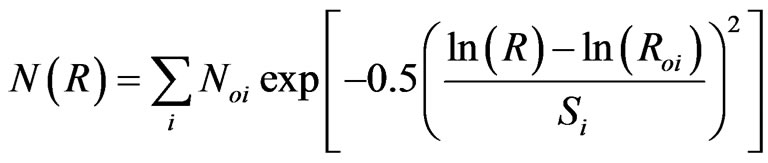 (3)
(3)
and
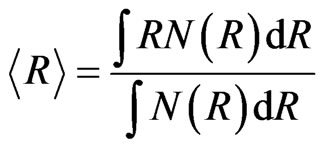 (4)
(4)
where No, Ro and S are the standard scaling factors, center and width parameters of this distribution type. The lognormal distribution was fitted in the q-range 0.1 - 1.1 nm–1 in Equation (1). The results of the fitting along with the observed data are shown in Figure 2.
The average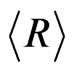 , of the particles calculated from the fitted parameters comes out to be 9.62 nm for the nanocrystalline commercial DEGUSSA P25 material as shown in Figure 3 from the number distribution function curve determined using the spherical model calculations
, of the particles calculated from the fitted parameters comes out to be 9.62 nm for the nanocrystalline commercial DEGUSSA P25 material as shown in Figure 3 from the number distribution function curve determined using the spherical model calculations
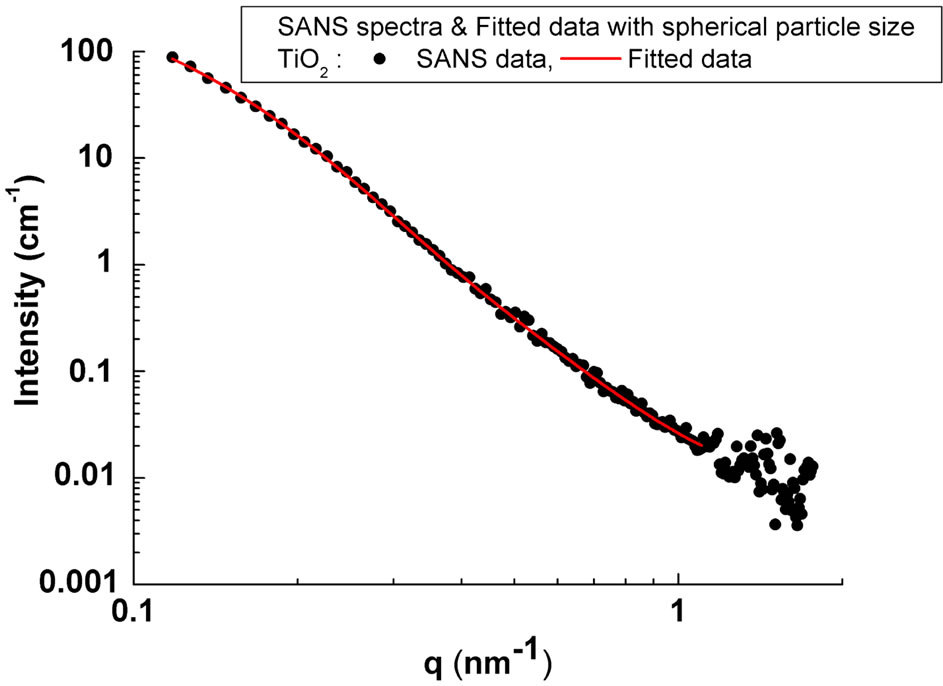
Figure 2. 1-D SANS Pattern averaged from the 2-D pattern for the nanocrystalline TiO2 powder sample; (solid lines shows spherical model fitting to the observed data).
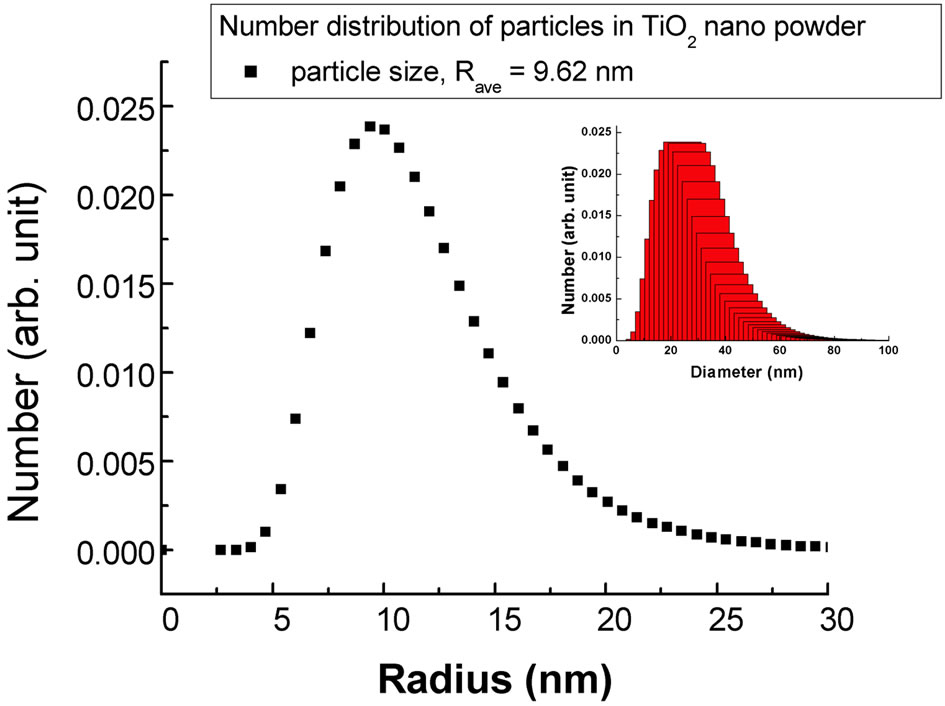
Figure 3. Particle size distribution for the nanocrystalline TiO2 powder sample (Inset shows the true particle diameter distribution).
mentioned above. Similar approached was used to estimate the size and microstructure of precipitates affected by coiling temperature in ultra low carbon steel in [18].
The size distribution was calculated in terms of the true particles diameter by the formula given below as mentioned in [19] and is shown in the inset of Figure 3.
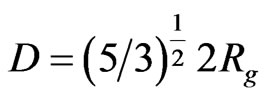 (5)
(5)
The average diameter of the spherical particle comes out to be 24.82 nm from SANS results, which corresponds well with the particles size determined by SunJae Kim, et al. [20] using transmission electron microscopy technique.
4. Conclusions
For nanocrystalline DEGUSSA P25, TiO2 powder the SANS results revealed that the average particle size of 24.82 nm agrees well with the earlier results of TEM. The XRD pattern proved coexistence of anatase and rutile phases with ratio of 75% and 25% respectively. Furthermore, the results demonstrates the power and utility of the SANS technique in characterizing microstructure, particle size, and distribution in nanocrystalline materials.
5. Acknowledgements
The authors are thankful to Mr. Chang-Hee Lee, B. S. Seong and Shin Enjoo of Neutron Scattering Division, KAERI, for their help in the SANS experiment and and data analysis. Their local hospitality is also highly acknowledged.