Carbon Sequestration under Warm Season Turfgrasses in Home Lawns ()
1. Introduction
Understanding the role of soils as a sink or a source for Carbon on a global scale is critical for evaluating changes in atmospheric carbon dioxide (CO2) concentration [1] . Land use change can alter soil C pools and can have a significant effect on the C balance between soils and the atmosphere. For example, conversion of forest to row crops results in depletion of soil organic carbon (SOC) by an average of 35% [2] . Trumbore et al. [3] found that when tropical dry forest in eastern Amazonia was converted to pasture, it lost an estimated value of 130 kg SOC ha−1・yr−1 from the top 10 cm of soil profile. In another part of eastern Amazonia, when moist tropical forest was converted to pasture, it lost about 300 kg SOC ha−1・yr−1 within the top 40 cm of soil [4] . In addition, rates of SOC sequestration under a subtropical wet forest plantation were higher than under a cool temperate-zone pine plantation, with an average accumulation of 338 kg C ha−1・yr−1 [5] . However, perennial grasses are more effective than woody plants at sequestering C in soil [5] . Baskin and Binkley [6] reported that significant changes in C sequestration were found when sugarcane fields were converted to fast growing eucalyptus plantations. After 10 - 15 years, SOC increased under eucalyptus in Hawaii by an average of 19.4 Mg・ha−1 in the top 55 cm of soil. Thus, the amount of organic C stored in soils varies with ecosystems and land use change [7] .
Most of the work on C sequestration has been conducted in row crops or forest eco- systems. There has been no work done on cool-season turf grasses, which indicated that golf course soils and home lawn soils sequestered carbon, but such C storage was offset by C emissions from fuel combustion, N fertilizer and irrigation [8] and [9] . Carbon sequestration in turfgrass may also be different from row-crop or forests because intensive management practices such as fertilization, irrigation, mowing and use of fuel-us- ing power equipment affect C flux [10] . Little research has focused on contributions from warm season turfgrasses such as bermudagrass, centipedegrass, and zoysiagrass. The turfgrass sector is a large industry with a sizable impact on the landscape of urban environments. The economic impact of turfgrass has been estimated to be $62.2 billion in the U.S., in 2005 [11] . Nationally, the turfgrass industry generates 822,849 jobs, $37.7 billion in labor income, and $2.4 billion in indirect business taxes [11] .
Milesi et al. [12] estimated the total U.S. turfgrass area to be 163,800 ± 35,850 km2. Turfgrass cover in the U.S. is rapidly expanding because of increasing urbanization and the addition of approximately 675,000 ha of residential property each year [13] . These areas of extensive grass cover could have a significant effect on soil C sequestration. For example, soils developed under temperate grassland sequestered a C mass of 192 Mg・ha−1 compared to 127, 118, and 56 Mg・ha−1 for soils developed under cultivated agriculture, temperate forest, and desert scrub, respectively [14] .
A later study evaluated C sequestration under different turfgrass species [15] . Cool season grasses such as fine fescue (Festuca rubra L.) (both irrigated and non-irrigated), Kentucky blue grass (Poa pratensis L.) (irrigated), and creeping bent grass (Agrostis stolonifera L.) (irrigated) were evaluated for changes in SOC, soil C sequestration and soil organic C decomposition. Four years after establishment, approximately 17% to 24% of SOC (0 - 10 cm depth) was contributed by the established turfgrass. Increases in SOC differed with turfgrass species and irrigation, with irrigated fine fescue having the highest SOC, estimated to be 3.4 Mg C ha−1・yr−1. All turfgrass species sequestered C during the first four years after turf establishment, with the highest rates for irrigated fine fescue and creeping bent grass [15] .
There is a limited study which examines C dynamics in the long-term, non-tilled setting with warm-season turfgrass management. Warm season turfgrasses (when grown in the southeast U.S.) are additionally novel in that they are dormant or quasi-dormant after a severe frost, regaining color and growth in the spring. Also, many of warm-sea- son turfgrass species grow via above-and below-ground stem structures (stolons and rhizomes, respectively), and these structures affect carbohydrate storage within the plant, which may further affect C storage. Thus, there is a need to evaluate C dynamics in warm-season turfgrass lawns in the southeast U.S. The objective of this study was to study C sequestration in warm-season turfgrasses managed in the home lawn environment, examining this sequestration as affected by grass species.
2. Materials and Methods
2.1. Study Sites
This two-year study (2012 and 2013) was conducted on randomly selected home lawns located in Auburn, AL (32.598˚N, 85.481˚W). Every lawn was within 1.6 km radius from other locations, and all lawns were of a similar age (the neighborhood in which the lawns were sampled largely had houses that dated to the 1950s and 1960s). All lawns were grown under similar soil type and environmental conditions including rainfalls and temperatures. In addition, they represent the most common turfgrasses in the southeastern U.S. From a survey of the homeowners, none of the lawns were new, and the averaged estimated age of the sampled lawns was more than 20 years. In all cases the soil type was a Marvyn loamy sand soil (fine-loamy, kaolinitic, thermic Typic Kanhapludult). Three turfgrass species were included in the study: bermudagrass, centipede grass and zoysiagrass. Cultivars were most likely: “common” centipede grass (all 6 sites), “Tifway” hybrid bermudagrass (all 6 sites) and “Emerald” (5 sites) or “Meyer” (1 site) zoysiagrass, all typical and available cultivars for 20-year old home lawns. Six lawns of each turfgrass species were sampled, for a total 18 lawns.
For this study, we had no control over mowing, clipping removal or fertilization pro- cedures for these lawns. However, a brief interview was conducted with each homeowner, and the following general management strategy was found for each lawn. Of the 18 lawns, eight were managed using the services of a home-lawn care company (2 centipedegrass, 3 each zoysiagrass and bermudagrass), with a total of 4 different companies for those lawns. All the other lawns were managed by the homeowners. Height of cut was determined by measuring verdure at harvest and was as follows: bermudagrass and zoysiagrass both at 5.0 to 6.3 cm, and centipedegrass and 6.3 to 7.5 cm. All mowing was done weekly using a rotary mower with clippings removed (in Auburn, AL clippings may still be piled at the curb for pickup, and most homeowners still engage in this practice). In all 2 zoysiagrass lawns and 1 bermudagrass lawn were irrigated using an installed in-ground system, with irrigation applied to supply water if drought occurred. All other lawns were rain fed. Selected sampled lawns were in an older and long established neighborhood, and in-ground irrigation is not common in these older lands- caped lawns. For all lawns, weed control consisted of one fall application of a pre- emergent herbicide for general control of winter annual weeds (primarily Poa annua), plus one application of a summer post-emergent broadleaf herbicide as needed. Last, fertilization was almost exclusively nitrogen applied as urea (46-0-0) or a mixed blend (10-10-10 or 13-13-13, N-P2O5-K2O) with a total yearly N rate of 9.8 g N m−2・yr−1 (applied as split applications in May and July, typically) for bermudagrass and zoysiagrass. Five of the centipedegrass lawns were never fertilized in the two year period, and the sixth received one application of 4.9 g N m−2・y−1.
2.2. Turf and Soil Sampling Procedures
Each lawn was sampled twice a year, in the summer (July) and in the winter (January), when the lawns were not growing or dormant. At each sampling six samples (5.0 cm diameter to a final depth of 20 cm) were collected using a hand-sampler from each lawn, with the samples taken randomly from each lawn. Fresh samples were taken to the laboratory, where they were immediately separated into tissue and soil components, as follows. Three of the samples were used for tissue sampling, with that sample separated into: 1) above ground growth (verdure) plus thatch (the intermingled live and dead plant tissue just below the shoots and above the soil), 2) stems (stolons and/or rhizomes), and, 3) roots. The remaining three samples were used for soil analysis, with those samples separated into the depth increments 0 - 5, 5 - 10, and 10 - 20 cm.
Soil samples were air dried and then ground to pass a 100-mesh sieve. Those samples were used for organic C analysis. Tissue samples were oven dried at 50˚C for 48 hr and then ground (by hand) to pass a 100 mesh sieve for organic C analysis. Initial pre-ex- periment assays with harvested tissue indicated that the previously mentioned drying temperatures and times result in a constant weight of dry tissue. Total C concentration was determined on finely ground oven-dried stem (rhizomes, stolons, and roots), above-ground tissue of turfgrass plus thatch and soil samples via dry combustion using a LECO TruSpec CN (LECO Corp., St. Joseph, MI).
2.3. Bulk Density
Bulk density was determined from core samples collected with a slide hammer (AMS, Inc., Sampling Equipment, American Falls, ID), with adjustment for rock content using the USDA procedure [16] .
2.4. Carbon Sequestration Calculation
Sequestered C was calculated as described by [17] as follows:
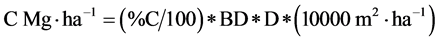
where:
% C = Mean percent of carbon content in soil
BD = Mean bulk density (in Mg・m−3)
D = Soil depth (m)
Stored C in biomass was calculated using C% and dry mass as follows:
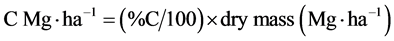 .
.
2.5. Data Analysis
Differences in C sequestration due to grass species were determined via analysis of variance using the mixed model procedure (SAS Institute Inc., Cary, NC). Season of measurement and year were considered as repeated measures; depth of each lawn used as the main variable and fixed effect. Denominator degrees of freedom were calculated using the Kenward-Roger option. Means were compared using Pairwise Multiple Comparison Procedures (Tukey’s Test) at P < 0.05.
3. Results and Discussion
Neither year nor season (winter or summer) had a significant effect on soil C in the year × grass × depth (P = 0.3018), year × depth (P = 0.1212), season × depth (P = 0.3114), or season × grass × depth (P = 0.9268) interaction. This indicates that, within our relatively narrow two-year sampling period, soil C was relatively stable across year and month of sampling. The interaction of grass × depth was always significant (P < 0.0001), indicating that grass species sequestered C differently with depth. Regardless of sampling date and year, there were significant differences in soil C accumulation as affected by turfgrass species (P < 0.05). Among all turfgrass species, C sequestered in underlying soil was highest in zoysiagrass lawns and lowest in bermudagrass lawns. The amount of soil C stored in zoysiagrass lawns was estimated at 2.33 Mg・ha−1・yr−1, 2.5 times greater than measured in bermudagrass lawns (0.92 Mg・ha−1・yr−1) in the top soil surface (0 - 5 cm) (Figure 1). When soil C was summed for the three sampling depths zoysiagrass had greatest soil C, followed by centipedegrass, and then bermudagrass. Zoysiagrass is generally considered to be a turfgrass with higher shoot densitythan lawn-type hybrid bermudagrass, especially for the Emerald cultivar (a fine-textured zoysiagrass) which predominated in this study. Thus, the greater shoot density of zoysiagrass may have created more biomass and a greater potential pool of soil C in that turfgrass.
In the upper 20 cm of soil, significant variation in accumulated C among turfgrass species was observed (P < 0.0001). For example, the amount of soil C stored (0 - 20 cm) in zoysiagrass lawns was 5.14 ± 0.21 Mg・ha−1・yr−1, compared to 1.8 ± 0.11 and 3.7 ± 0.14 Mg・ha−1・yr−1 in bermudagrass and centipedegrass lawns, respectively (Figure 1). Carbon stored in soil under zoysiagrass was almost three times greater than that found in soil under bermudagrass. The amount of carbon stored in bermudagrass soils was comparable to the 1.4 Mg C ha−1・yr−1 stored in the top 20 cm of soil under ornamental lawns [18] . The reason behind higher accumulation of organic C under zoysiagrass
![]()
Figure 1. Carbon sequestration (Mg・ha−1・yr−1) in soil as affected by turfgrass species and soil depth. Bars show the standard error of the mean.
may again be explained by a typically greater shoot density as compared to bermudagrass and centipedegrass, which might have resulted in higher C sequestration in zoysia grass compared to bermudagrass and centipedegrass.
Plant C is added to soil by deposition and decomposition of aboveground plant parts (litter decomposition) and belowground root exudates [19] . In our study, carbon stored in roots was less than that measured in the surrounding soil, and was greatest in the top layer (0 - 5 cm) (Figure 2(a)). Major sources of SOC accumulation are from belowground plant root activities [20] and aboveground biomass decomposition. Root densities of turfgrasses typically decline with depth, while decomposition of clippings and stems at or near the soil surface enriches the upper soil with C. These combined effects demonstrate why highest C accumulation in roots was measured in the 0 - 5 cm layer, with declines with depth (Figure 2(a)). In all cases C sequestration in roots decreased with depth. Greatest C storage was in zoysiagrass roots, followed by centipedegrass and then bermudagrass.
The intensity of root exudates varies with different compositions of plant tissues [21] . For example, more than 45% of the C assimilated by trees is ultimately transported belowground via root growth and root exudates, making soil a significant sink of C [22] . Reyes-Reyes et al. [23] and Yelenik et al. [24] observed an increase in belowground C stock when grass dominated ecosystems were invaded by a tree population in the central highlands of Mexico. Similarly, silvopasture systems were observed to have a greater accumulation of C in soil when compared with adjacent pastures (tree free) in Florida [25] .
Carbon sequestration in roots significantly (P ≤ 0.05) varied by depth among grass species with season (P = 0.0010) and year (P < 0.0001). The two-way interactions grass ×
![]()
Figure 2. Effect of grass species and soil depth on carbon sequestration (kg・ha−1・yr−1) in grass root (a), and, weight of root biomass (b). Bars show the standard error of the mean.
depth and grass × year significantly (P < 0.0001) affected C accumulation in the roots. The amount of C stored in grass roots in the 5 to 10 cm layer was higher in the zoysiagrass lawns (69.7 ± 2.7 kg・ha−1・yr−1) compared to bermudagrass and centipedegrass lawns (9.7 ± 1.2 and 18.6 ± 0.7 kg・ha−1・yr−1) respectively) (Figure 2(a)). A decline in C from roots was observed as sampling depth increased.
Differences in accumulated C content among grasses can be explained by the differences in grass root biomass (Figure 2(b)) and, possibly, anthropogenic activities such as clipping frequency and fertilization, which impact the above ground biomass, affecting C transported belowground and partitioned in root growth. When actual root biomass was measured (Figure 2(b)), both centipedegrass and zoysiagrass had greater rooting at all depths. The difference in rooting density at lower depths is probably the reason for the difference in C accumulation. The difference in SOC between the top and bottom soil layer was the highest (75%) in centipedegrass. This difference may be due to the fact that centipedegrass is the sole turfgrass in this study that has only stoloniferous growth (above-ground stems), and not both stolons and rhizomes (above- and below-ground stems, respectively). Thus, extensive rhizomes in zoysiagrass and bermudagrass may have contributed to SOC deeper in the profile due to decomposition of rhizomes.
Above ground biomass had significant variation in accumulated C among selected turfgrasses with year (P < 0.0001). The two-way interaction between grass × year was very significantly (P = 0.0066) affected by C accumulation in the aboveground biomass. For example, C sequestered in the aboveground biomass of zoysiagrass was the highest, 0.11 ± 0.008 Mg・ha−1・yr−1 compared to 0.088 ± 0.008 and 0.056 ± 0.018 Mg・ha−1・yr−1 in bermudagrass and centipedegrass, respectively (Figure 3(a)). Differences in sequestered C in verdue were a function of aboveground biomass (Figure 3(b)).
Significant variation in accumulated C among turfgrass species was found in thatch and stem tissue (P < 0.0001). The two-way interaction grass × year and grass × season and the three-way interaction grass × year × season significantly (P < 0.0001) affected C accumulation in stems. For example, C sequestered in the thatch layer + stolons/rhi- zomes of zoysiagrass increased from 0.70 ± 0.03 (January 2012) to 0.74 ± 0.03 Mg・ha−1 (July 2103) a 6% increase. The amount of C accumulated in stolon/rhizome of bermudagrass increased from 0.33 ± 0.02 (January 2012) to 0.38 ± 0.03 Mg・ha−1・yr−1 (July 2103), a 13% increase. Carbon stored in centipedegrass (thatch layer + stolons only) was 0.10 ± 0.01 Mg・ha−1・yr−1, which is two times greater than in zoysiagrass lawns, which was 0.05 ± 0.001 Mg・ha−1・yr−1 (Figure 3(c)). Differences in accumulation of C may be explained by the differences in stolon/rhizome biomass under all grasses (Figure 3(d)). Statistical analyses indicated that turfgrass species, season and year sampling significantly (P ≤ 0.05) affected total (including soil, roots, thatch + rhizome/stolon, and aboveground mass) C sequestration in the soil profile. The two-way interaction grass × year affected significantly C accumulation in the top 20 cm of soil depth (P = 0.04). Zoysia grass had highest levels of sequestered C with a total (soil, roots, thatch + rhizome/stolon, and aboveground mass) value of 5.54 ± 0.21, compared to 2.09 ± 0.11 and 4.23 ± 0.14 Mg・ha−1・yr−1 under bermudagrass and centipedegrass systems, respectively. Accumulated C content differences among grasses can be explained by the differences in grass biomass and human activities such mowing frequency, fertilization or clipping removal, in addition to environmental factors including soil and air temperature, soil moisture, and structure.
4. Conclusions
Grassland is a major component of the terrestrial ecosystem, comprising 27% of the total US area [26] . These areas are vital to global C sequestration, and may act as sources or sinks for C at a significant level [27] . Due to that large area of land covered by grass, even a very small change in management practices to reduce C emissions may be an
![]()
Figure 3. Effect of grass species on carbon sequestration (Mg・ha−1・yr−1) in aboveground (a) and thatch layer + rhizome/stolons (c), and weight of aboveground biomass (b) and weight of thatch layer + rhizome/stolon (d). Bars show the standard error of the mean.
important contribution to the global C sequestration level. For example, CO2 emission was reduced in bermudagrass by reductions in nitrogen fertilization [28] and [29] . Carbon storage in lawns could also be increased by reducing mowing, and returning clippings. This study found that zoysiagrass had greater above- and below-ground biomass that resulted in greater C inputs to the soil than other warm-season turfgrasses, likely due to the individual or combined effects of species and plant density. However, more research is needed on inputs such as litter quality and quantity, mowing, irrigation and clipping management to better quantify C flux in home lawns.