Zinc Chloride Protects against Streptozotocin-Induced Diabetic Nephropathy in Rats ()
Received 12 July 2016; accepted 26 August 2016; published 29 August 2016

1. Introduction
Diabetes is a serious health hazard currently affecting more than 220 million people worldwide and expected to affect 400 million by 2030 [1] [2] . Diabetes being a metabolic disorder produces in the long run, cell dysfunction in almost all organs in the body. The most serious complications of diabetes are coronary artery disease, nephropathy, retinopathy, and neuropathy. Oxidative stress is thought to play a major role in the development of most of these complications [3] - [5] . Oxidative stress may occur when antioxidant mechanisms are not working properly as in dietary deficiencies of vitamin E, vitamin C or the essential elements like selenium, zinc, and manganese among others. The later elements are essential components of the antioxidant enzymes glutathione peroxidase, superoxide dismutase and catalase [6] - [8] . Another important cause of oxidative stress is the excessive endogenous production of free radicals by diseases progression as in diabetes mellitus and cancer [6] .
Diabetic nephropathy is a severe, chronic complication of diabetes mellitus, and it is the leading cause of end-stage renal failure in diabetic patients [9] . It results from the combined effects of various genetic and environmental factors. Elevated glucose and cholesterol levels, increased production of inflammatory cytokines are the predisposing factors for the progression of renal damage in diabetic nephropathy [10] . Elevated glucose levels were recognized as a pathogenic factor of chronic diabetic complications by generating reactive oxygen species (ROS) and attenuating the antioxidative machinery via glycation of the antioxidant enzymes [11] . The major ROS sources in the diabetic nephropathy were: autoxidation of glucose, the activation of polyol pathways, mitochondrial respiratory chain deficiencies, xanthine oxidase activity, NAD(P)H oxidase, advanced glycation end products (AGEs) and nitric oxide synthase (NOS) [12] . The increased oxidative stress leads to injuries of the glomeruli [13] , tubular interstitial tissue [14] and vasculature [15] . It is implicated in the mesangial expansion of extracellular matrix, and results in increased glomerular filtration rate, urine protein excretion, progression of glomerular sclerosis and tubular interstitial fibrosis [16] - [18] . Thus antioxidative therapy may be an effective way to treat diabetic nephropathy [19] . In addition, agents having both antioxidant and hypoglycemic effects might have promising protective actions against diabetic nephropathy [20] .
Zinc is an essential component of numerous proteins which play crucial roles in growth and development. It showed potent antioxidant and anti-inflammatory properties and was involved in the defense against oxidative stress [21] [22] . Diabetic patients often suffer from Zinc deficiency at the late stage, particularly in the patients whose glucose was poorly controlled [23] - [26] . In addition, zinc has been reported to exert antidiabetic effects in various experimental models [27] . Thus, it seems that Zinc is a proper mineral supplement in diabetic patients. The STZ-diabetic animal model was selected for this study as it induces nephropathy analogous to the early stages of clinical nephropathy [28] [29] . STZ displayed pancreatic beta-cell toxicity via different mechanisms including targeted uptake of STZ in beta cells by Glut2 receptors [30] and increased oxidative stress due to NO release and ROS production [31] [32] .
The aim of the present study was to examine the effect of Zinc Chloride treatment on the progression of renal biochemical changes in STZ-induced diabetic rats. It was designed to determine whether therapeutic intervention with Zinc Chloride would prevent the onset and progression of renal complications or not as compared with Gliclazide, a reference antidiabetic. Gliclazide has been reported to protect against renal damage in glomeruli and the proximal and distal tubules in a diabetic rat model [33] .
2. Material and Methods
2.1. Animals
Adult Wistar rats of either sex weighing 160 - 200 g were obtained from the animal house of King Abdulaziz University, Jeddah, Saudi Arabia. The animals were maintained under controlled laboratory conditions (temperature (22˚C ± 1˚C), humidity (60% ± 5%), and a 12/12 h light/dark cycle).
The experimental protocol was approved by the Animal Care and Use Committee of the University of Umm Al-Qura, KSA. Maximum effort was made to minimize animal suffering.
2.2. Chemicals
Streptozotocin (STZ) was purchased from Sigma-Aldrich (USA). Zinc Chloride was purchased from Qualikems (India). Reduced glutathione and lipid peroxides detection kits were purchased from Biodiagnostic (Egypt). Creatinine, urea, and albumin assay kits were purchased from Salucea, Dutch technology in life science (Netherlands). Nitric oxide colorimetric assay kit was purchased from Biovision (USA). Other used chemicals were of the highest analytical grade.
2.3. Experimental Design and Treatment Protocol
Diabetes was induced by intraperitoneal injection of STZ dissolved in 0.1 mol/L sodium citrate buffer (pH = 4.0) at a dose of 65 mg/kg body weight [10] . Fasting, tail-vein, blood glucose was measured three days after injection by using one touch glucometer (Yi Cheng, BeiJing, China), and rats that have a glucose level over 250 mg/dl were considered diabetic [34] . The normal control group received citrate buffer.
Diabetic rats were divided into 3 groups of 8 animals each. The first group served as diabetic control. The second group rats received a daily IP injection of 5 mg/kg Zinc Chloride for one month. The third group, rats received a daily IP injection of 10 mg/kg gliclazide for one month [35] .
2.4. Tissue Collection and Preparation
On the 30th day, rats were fasted overnight. Blood pressure and heart rate were measured using Tail cuff (9), then rats were sacrificed under light ether anesthesia. The blood samples were collected, centrifuged and serum was kept frozen for measuring: glucose, insulin, urea, albumin, and creatinine.
The two kidneys were isolated and kept frozen for measuring: malondialdehyde (MDA), reduced glutathione (GSH) and nitric oxide (NOx).
2.5. Determination of Blood Pressure and Heart Rate
Rats blood pressure and heart rate were assessed by using CODATM (Kent Scientific, Torrington, CT, USA). Readings were taken for 20 cycles from each rat. Systolic and diastolic blood pressure were expressed as mmHg. Heart rate was expressed as beat/min.
2.6. Determination of Mean Arterial Pressure
Mean arterial pressure (MAP), is defined as the average pressure in arteries during one cardiac cycle. It is considered a better indicator of perfusion to vital organs than systolic blood pressure (SBP). MAP was calculated using the following equation and expressed as mmHg
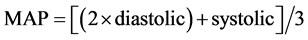
2.7. Determination of Blood Glucose Level
Fasting serum glucose level was determined colorimetrically according to the method of Trinder (1969) using a commercial reagent kit.
2.8. Determination of Blood Insulin Level
Fasting serum insulin level was determined using a commercial ELISA kit (Li KaShing Faculty of Medicine, The University of Hong Kong, AIS).
2.9. Determination of Kidney Function
Serum creatinine, serum urea, and serum albumin levels were determined as previously described using commercial reagent kits (Salucea, Dutch technology in life science, Netherlands).
2.10. Determination of Kidney Lipid Peroxides Content
Lipid peroxidation was determined as described previously by [36] and according to manufacturer procedures of the assay kit (Biodiagnostic) and were expressed as mg %, thiobarbituric acid reactive substances (TBARS) content, estimated as malondialdehyde (MDA). The absorbance was measured at 520 nm and expressed as mmol/g. tissue.
2.11. Determination of Kidney GSH Content
Reduced glutathione (GSH) was determined as described previously by [37] and the content was estimated following manufacturer’s procedure of the assay kit (Biodiagnostic). The method is based on the reduction of 5,5’ dithiobis (2-nitrobenzoic acid) (DTNB) with glutathione to produce a yellow compound; the reduced chromogen is directly proportional to GSH concentration, and the absorbance was measured at 405 nm and expressed as mmol/g. tissue.
2.12. Determination of Kidney Total Nitrate and Nitrite Contents
Total nitrate/nitrite (NOx) contents were measured using Nitric Oxide ( /
/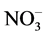 ) Colorimetric Assay kit (Biovision) according to manufacturer procedures. The method is based on the enzymatic conversion of nitrate to nitrite, utilizing Nitrate Reductase, followed by Griess reagent and the formation of a deep purple azo compound which absorbs visible light at 540 nm. The amount of the azo chromophore accurately reflects nitric oxide amount in the samples. Results were expressed as nmol/g. tissue.
) Colorimetric Assay kit (Biovision) according to manufacturer procedures. The method is based on the enzymatic conversion of nitrate to nitrite, utilizing Nitrate Reductase, followed by Griess reagent and the formation of a deep purple azo compound which absorbs visible light at 540 nm. The amount of the azo chromophore accurately reflects nitric oxide amount in the samples. Results were expressed as nmol/g. tissue.
2.13. Statistical Analysis
The data were presented as means ± standard error (S.E.). Statistical analysis was performed using SPSS statistical software (version 16). Comparisons between different groups were carried out using one-way analysis of variance (ANOVA) followed by Tukey-Kramer multiple comparisons test. The Differences were considered statistically significant at p < 0.05.
3. Results
3.1. The Effect of Zinc Chloride on Blood Glucose and Insulin Levels
The results of the present study confirmed that administration of STZ in rats showed a significant 3.3-fold increase in serum glucose level and a significant decrease in serum insulin levels to 39% compared to normal control group, while treatment with Gliclazide restored normal serum levels of glucose and insulin. Similarly, Zinc Chloride significantly decreased the elevated blood glucose levels by 51% as compared to diabetic control group. In addition, Zinc Chloride administration significantly increased insulin levels to 211% as compared to STZ- induced diabetic rats (Figure 1(a) & Figure 1(b)).
3.2. The Effect of Zinc Chloride on Blood Pressure and Heart Rate of STZ-Induced Diabetic Nephropathy in Rats
Zinc Chloride administration was capable of normalizing the elevated blood pressure in DN rats. Results showed a significant decrease in systolic and diastolic blood pressure, heart rate and mean arterial pressure to 88.6%, 86.3%, 95% and 87.1% respectively, compared to diabetic control group (Table 1). Similar results were obtained by Gliclazide treatment.
3.3. The Effect of Zinc Chloride on Renal Function of STZ-Induced Diabetic Nephropathy in Rats
To explore the effects of Zinc Chloride treatment on renal function, the levels of BUN, serum creatinine, and Albumin were investigated in STZ-induced diabetic rats. Compared with the diabetic control group, Zinc Chloride group showed a significant decrease in BUN and serum creatinine by 21.9% and 30% respectively (Figure 2(a) & Figure 2(b)). On the other hand, Zinc Chloride treated group did not show a significant change in albumin level compared to the DN.
![]() (a)
(a)![]() (b)
(b)
Figure 1. The effect of Zinc Chloride on blood glucose and insulin levels of STZ-induced diabetic nephropathy in rats. (a) Blood glucose and (b) Insulin. Diabetic nephropathy was induced by a single intraperitoneal injection of STZ dissolved in 0.1 mol/L sodium citrate buffer (pH = 4.0) at a dose of 65 mg/kg body weight, whereas the control group received the same volume of physiological saline. Zinc Chloride group, rats received 5 mg/kg Zinc Chloride for one month. Data were expressed as mean ± S.E.M (n = 8). @Significant difference from diabetic control group at p < 0.05, bSignificant difference from Gliclazide group at p < 0.05. Zn; Zinc Chloride, Glic; Gliclazide.
![]()
Table 1. The effect of Zinc Chloride administration on the blood pressure and heart rate of STZ-induced diabetic nephropathy in rats.
![]() (a)
(a)![]() (b)
(b)![]() (c)
(c)
Figure 2. Zinc Chloride ameliorates kidney functions of STZ-induced diabetic nephropathy in rats. (a) Blood urea nitrogen (BUN). (b) Serum creatinine (sCr). (c) Albumin. Diabetic nephropathy was induced by a single intraperitoneal injection of STZ dissolved in 0.1 mol/L sodium citrate buffer (pH = 4.0) at a dose of 65 mg/kg body weight, whereas the control group received the same volume of physiological saline. Zinc Chloride group, rats, received 5 mg/kg Zinc Chloride for one month. Data were expressed as mean ± S.E.M (n = 8). @Significant difference from diabetic control group at p < 0.05, bSignificant difference from Gliclazide group at p < 0.05. Zn; Zinc Chloride, Glic; Gliclazide.
3.4. The Effect of Zinc Chloride on Kidney’s Oxidative Stress Biomarkers
In the diabetic control group, STZ evoked oxidative stress and diminished cellular antioxidant capacity, which has been intimately linked to diabetic nephropathy [38] . Oxidative stress was indicated by the elevated levels of MDA (144.4%) with the concomitant decline of serum GSH (70.5%) compared to normal control group (Figure 3(a) & Figure 3(b)). Treatment Gliclazide or Zinc Chloride significantly protected against oxidative stress by the reinstatement of GSH and MDA as compared to diabetic control group.
![]() (a)
(a)![]() (b)
(b)
Figure 3. Zinc Chloride boost antioxidant defenses in rats with STZ-induced diabetic nephropathy. (a) Malondialdehyde; MDA. (b) Reduced glutathione; GSH. Diabetic nephropathy was induced by a single intraperitoneal injection of STZ dissolved in 0.1 mol/L sodium citrate buffer (pH = 4.0) at a dose of 65 mg/kg body weight, whereas the control group received the same volume of physiological saline. Zinc Chloride group, rats, received 5 mg/kg Zinc Chloride for one month. Data were expressed as mean ± S.E.M (n = 8). @Significant difference from diabetic control group at p < 0.05, bSignificant difference from Gliclazide group at p < 0.05. Zn; Zinc Chloride, Glic; Gliclazide.
3.5. The Effect of Zinc Chloride on Kidney NO Content
The impact of Zinc Chloride administration on kidney defenses such as NO was also addressed. NO levels were increased in the diabetic control group (149.3%) as compared to normal control group. Gliclazide or Zinc Chloride were able to restore NO level suggesting their role in attenuation of DN (Figure 4). It was worth noting that Zinc Chloride was more effective than gliclazide in normalizing kidney NO content.
4. Discussion
Diabetic nephropathy (DN), as a component of diabetic vascular diseases, worsens most diabetic complications. In particular, the risk of morbidity and mortality from cardiovascular disease is increased several folds [39] . DN is the leading cause of end-stage renal disease (ESRD) in diabetic patients [40] . It affects about 40% of diabetic patients worldwide. Tight control of glucose level has been reported to reduce both the development and progression of diabetic nephropathy, retinopathy and cardiovascular disease [41] [42] . Agents possessing both hypoglycemic and antioxidant properties were expected to protect against diabetic nephropathy [20] .
The findings of the present study indicated that Zinc Chloride blunted the increment in serum glucose, while increased serum insulin levels in diabetic control rats. Consistent with our work, several studies showed the ability of Zinc Chloride to control hyperglycemia [10] [43] . In addition, our results showed a significant increase in the mean arterial blood pressure in the diabetic control rats. These results are in accordance with the observations of Dhein et al. 2000, who reported a marked increase in MAP in diabetic rats [44] . On the other hand, treatment with Gliclazide or Zinc Chloride produced a significant reduction in the elevated MAP, which was in contrast to Moreau et al. 1994, who reported that the glibenclamide-induced blockade of vascular potassium channels caused a vasoconstriction in the systemic and splanchnic vascular beds and hence an increase in blood pressure. [45] . Williams et al. in 1998, reported that night-time systolic blood pressures were significantly higher during glibenclamide treatment than it was with placebo [46] . In addition, Kulkarni et al., in 2002, reported that glibenclamide significantly prevented alloxan-induced hyperglycemia and hypoinsulinemia, but it failed
![]()
Figure 4. Zinc Chloride restores NO defenses in rats with STZ-induced diabetic nephropathy. Diabetic nephropathy was induced by a single intraperitoneal injec- tion of STZ dissolved in 0.1 mol/L sodium citrate buffer (pH = 4.0) at a dose of 65 mg/kg body weight, whereas the control group received the same volume of physiological saline. Zinc Chloride group, rats, received 5 mg/kg Zinc Chloride for one month. Data were expressed as mean ± S.E.M (n = 8). @Significant difference from diabetic control group at p < 0.05, bSignificant difference from Gliclazide group at p < 0.05. Zn; Zinc Chloride, Glic; Gliclazide.
to alter hypertension [47] .
Elevated serum levels of urea and creatinine, due to interstitial atrophy and vasodilated atrophic changes in the glomeruli and tubules, have been observed during the progression of diabetes and were known as diabetic nephropathies [48] . Therefore, those were used for the observation of DN’s occurrence and progression [49] . An improvement in these abnormal changes is considered direct evidence of an improvement in DN [50] . The present data showed that administration of Zinc Chloride decreased the serum urea and creatinine levels in diabetic rats (Figure 2). Together the data show for the first time the effect of Zinc Chloride in controlling DN.
In our attempt to understand the mechanism underlying the effect of Zinc Chloride in controlling DN, we assessed some of the oxidative stress parameters. Zinc Chloride accentuated serum GSH levels while attenuated the increased levels of MDA and kidney NO, boosting the antioxidant defenses of the kidney. In agreement with the present findings, a study reported the effect of Zinc Chloride on improving MDA and total antioxidant capacity in diabetic rats [27] . Another study also showed the beneficial effects of Zinc Chloride in controlling hyperglycemia thus preserving liver architecture and ameliorating NO, MDA, superoxide dismutase (SOD) and GSH levels [10] . Our results, showing the antioxidant capacity of Zinc Chloride, are in accordance with Li et al., in 2014, who reported the role of Zinc Chloride in preventing renal oxidative damage via upregulation of nuclear factor-erythroid 2-related factor (Nrf2) [43] .
5. Conclusion
The present study demonstrates for the first time the potential of Zinc Chloride in the management of diabetic nephropathy and suggesting it as a beneficial supplement for diabetic patients. However, further investigations would be required to elucidate fully the mechanisms involved in reducing diabetic nephropathy and intricate interplay among different metabolic pathways.
Acknowledgements
This work was supported by a research grant number 43410011 from Scientific Research & Islamic Heritage Institute, Umm Al Qura University, Makkah, Saudi Arabia.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Abbreviations
DM: Diabetes mellitus,
DN: Diabetic nephropathy,
GSH: Glutathione,
MDA: Malondialdehyde,
NO: Nitric oxide,
ROS: Reactive oxygen species,
sCR: Serum creatinine.

Submit or recommend next manuscript to SCIRP and we will provide best service for you:
Accepting pre-submission inquiries through Email, Facebook, LinkedIn, Twitter, etc.
A wide selection of journals (inclusive of 9 subjects, more than 200 journals)
Providing 24-hour high-quality service
User-friendly online submission system
Fair and swift peer-review system
Efficient typesetting and proofreading procedure
Display of the result of downloads and visits, as well as the number of cited articles
Maximum dissemination of your research work
Submit your manuscript at: http://papersubmission.scirp.org/
NOTES
![]()
*Corresponding author.