Effects of Bisphenol A on Testosterone Levels and Sexual Behaviors of Male Mice ()
1. Introduction
Bisphenol A (BPA, chemical structure: C15H16O2) is thought to be an environmental estrogen which generally exists in the environment [1] . Human may intake BPA at low dosage via diet, dust or skin in their daily activities [2] . BPA can be detected in urine, serum, placental tissue and even in the fetal liver [3] . Environmental exposure to BPA is associated with many disorders in humans including heart failure [4] , kidney diseases [5] and immune system dysfunction [6] , etc. BPA can suppress spermatogenesis and decrease semen quality by impairing meiosis, inducing apoptosis of spermatids, or depressing the function of hypothalamic-pituitary-gonadal axis (HPG axis) [7] . However, there have been few reports discussing the underlying effects of BPA on sexual functions. The aim of this study was to assess whether BPA could affect the level of testosterone in vivo and impair the sexual function of male mice.
2. Materials and Methods
2.1. Ethics Statement
Animals and experiment protocols in the present study were approved by the Sun Yat-sen University Institutional Animal Care and Use Committee. All animals received humane care, and all efforts were taken to minimize suffering. After the experiments, animals received euthanasia by CO2 inhalation.
2.2. Animals
Forty male C57BL/6 mice and 10 female C57BL/6 mice were purchased from the Animal Center of Sun Yat‑sen University (Guangzhou, China) and were maintained on a 12 h-day/12 h-night schedule (lights on from 19:00 to 07:00 h) at constant temperature (22˚C ± 1˚C) and humidity (60%). These animals were approximately 12 weeks of age and weighted 22 g - 25 g.
2.3. Drugs and Treatments
BPA and olive oil were purchased from Sigma.BPA was dissolved in olive oil before administration. All of the male mice were assigned into four equal groups (10 male mice per group). One group was treated with physiological saline intraperitoneally as the con- trol group, and the other three groups were treated with BPA via intraperitoneal injection at different dosages for 21 consecutive days (10, 50, 100 mg/kg per day respectively).
2.4. Tests of Sexual Behaviors
As depicted previously, the female mice were used as stimulus females in the test and were resected with double ovaries in advance [8] . One month later, each female mouse was pretreated with estradiol benzoate (50 μg, 48 h before testing, dissolved in 50 μl of peanut oil and injected intraperitoneally) and progesterone (500 μg, 5 h before testing, dissolved in 50 μl of peanut oil and also injected intraperitoneally) reach a state of estrous.
Sexual behaviors tests have been described previously [8] . The following items were recorded during the tests: mount latency, intromission latency, ejaculation latency, mount frequency, intromission frequency, andcopulatory efficacy (calculated as intromission frequency divided by mount frequency + intromission frequency). The sexual assays were conducted in a clean testing cage (40 cm × 26 cm × 21 cm) in a quiet room. Each mouse was placed individually into the testing cage for preadaptation. Fifteen minutes later, a pretreated female mouse was placed into this cage. If ten minutes passed without an intromission, another pretreated female mouse was put into this cage to replace the first female mouse. If the second female mouse failed to achieve intromission with the male mouse again within the next five min, a third pretreated female mouse was introduced for a final fifteen minutes period. Failure to achieve intromission within fifteen minutesor ejaculation within forty-five minutes from the beginning of the test resulted in termination of the experiment, and the maximum latency value of forty-five min was assigned for that behavior.
2.5. Weighting of Body and Reproductive Organs
After the examination of sexual behaviors, each of the male mice was weighed. Then, all of the animals were anesthetized with ether, and blood samples were obtained from the vena cava for the examination of serum testosterone. Immediately after blood sampled, bilateral testes, epididymides, and seminal vesicles were obtained and weighed.
2.6. Testosterone Examination
Serum testosterone was assayed by the enzyme-linked immunosorbent assay (ELISA) kit (R & D Systems, Minneapolis, MN, USA) following the manufacturer’s instructions. Intratesticular testosterone (ITT) concentrations were examined as previously described [8] . In brief, 50 mg testicular tissues from each mouse were homogenized by sonication (2 s × 20 s) in phosphate buffer solution (PBS) and then centrifuged at 10,000 g for ten minutes. Testosterone concentrations in the supernatants were also assayed using the ELISA kit depicted above. The outcomes were expressed as ng/g tissue.
2.7. Statistical Analysis
Results were presented as mean ± s.e.m. In all cases, P < 0.05 was thought to be statistically significant. One-way ANOVA for multiple group comparisons was employed to assess the significance of differences. All analytic results were performed using the Graph Pad Software package (GraphPad Software 6.0, La Jolla, CA, USA).
3. Results
3.1. Results of Sexual Behaviors Tests
As shown in Figure 1, the mount latency was lengthened at the dose of 100 mg/kg compared with the control (11.64 ± 2.67 min vs. 8.98 ± 1.40 min, P < 0.05). The intromission latency was lengthened at the dose of 50 mg/kg (20.28 ± 3.40 min, P < 0.05) and 100 mg/kg (20.13 ± 2.06 min, P < 0.05). No obvious differences of ejaculation latency were observed between the control group and experimental groups (P > 0.05). Although all of the three experimental groups had a downward trend in mount frequency, only the change at 100 mg/kg was statistically lower than the control (0.52 ±
![]() (a) (b)
(a) (b) ![]() (c) (d)
(c) (d)![]() (e) (f)
(e) (f)
Figure 1. Measures of sexual behaviors of the control group and BPA-treated groups. All values are mean ± s.e.m., *P < 0.05 compared with control group. (a) Mean latency of mount; (b) Mean latency of intromission; (c) Mean latency of ejaculation; (d) Mean frequency per minute of mount; (e) Mean frequency per minute of intromission; (f) mean copulatory efficacy.
0.15 numbers/min vs. 0.67 ± 0.13 numbers/min P < 0.05). Similarly, there was a decrease of intromission frequency and copulatory efficacy in the 100 mg/kg group compared with the control group (intromission frequency: 0.37 ± 0.12 numbers/min vs. 0.53 ± 0.17 numbers/min; copulatory efficacy: 0.40 ± 0.03 vs. 0.45 ± 0.02; both P < 0.05).
3.2. Effects of BPA on the Weight of Body and Reproductive Organs
As shown in Table 1, the control group and experimental groups did not exhibit differences in body weight after the treatment of BAP. There was a decrease in the testis weight of each BPA-treated group compared to that of the control group (the 10 mg/kg group, P < 0.05; the 50 mg/kg group, P < 0.05; the 100 mg/kg, P < 0.01). There was also a decrease in the relative testis weight in the groups at the dose of 100 mg/kg (P < 0.05). Only the epididymis weights and relative epididymis weights in the 100 mg/kg group were lower than the control group (both P < 0.05). Similarly, both the weight and the relative weight of seminal vesicle in groups at the dose of 100 mg/kg were significantly lower than the control group (both P < 0.05).
3.3. Changes in Serum Testosterone and ITT
As shown in Figure 2, the serum testosterone levels of these mice at a dose of 100 mg/kg decreased obviously after treatment with BPA (7.88 ± 1.62 ng/ml vs. 9.23 ± 1.42
![]() (a)
(a)![]() (b)
(b)
Figure 2. Effects of BPA on the levels of serum testosterone (a) and intratesticular testosterone (b). All values are mean ± s.e.m., *P < 0.05, **P < 0.01, compared with control group.
![]()
Table 1. Changes in body weights and reproductive organs (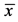 ± s.e.m).
± s.e.m).
Notes: BW: body weight; TW: testis weight; Relative TW: TW/BW × 100; EW: epididymis weight; Relative EW: EW/BW × 100; SW: weight of seminal vesicle; Relative SW: SW/BW × 100; *P < 0.05: statistical significance compared with control group; s.e.m: standard error of mean.
ng/ml, P < 0.05). Similarly, the ITT of the 50 mg/kg group and the 100 mg/kg group was both statistically lower than the control group (the 50 mg/kg group: 75.5 ± 7.18 ng/g; the 100 mg/kg group: 73.00 ± 9.57 ng/g; the control group: 85.10 ± 9.71 ng/g. P < 0.05, and P < 0.01, respectively). No changes were observes in other experimental groups compared with the control.
4. Discussion
In the present study, we selected adult male C57BL/6 mice as an animal model to test the effects of BPA on testosterone levels and sexual behaviors of male mice. Our results demonstrated that the administration of BPA could decrease the testosterone synthesis in vivo and suppress sexual functions of male mice.
It was reported that male health has been seriously threatened, and the environmental pollution was thought to be the main reason of this phenomenon [1] . BPA is a common environmental estrogen with endocrine Interference effect, and can affect multiple organs of human [4] - [6] . One research revealed that BPA can affect the function of thyroid gland and may disrupt the function of nuclear hormone receptors and their cofactors to disturb our internal hormonal environment [9] . Braun et al. suggested that prenatal exposure of BPA may be associated with externalizing behaviors in children of 2 years old, especially among female children [10] . They also reported that gestational BPA exposure can affect behavioral and emotional regulation domains at age 3, especially among girls [11] . An animal experiment reported that BPA exposure promotes a podocytopathy with proteinuria, glomerular hyperfiltration and podocytopenia [12] . Holladay et al. reported that BPA can raise the levels of many kinds of cytokine and induce developmental immunotoxicity [6] . As an environmental estrogen, BPA can affect the functions of reproductive system and lead to infertility. Fujimoto et al. suggested that BPA exposure in female patients may interfere with oocyte quality during in vitro fertilization [13] . Knez et al. investigated 149 couples undergoing in vitro fertilization or intracytoplasmic sperm injection procedure and concluded that urinary BPA concentrations in male partners of subfertile couples may influence semen quality parameters, but do not affect embryo development up to the blastocyst stage after medically assisted reproduction [14] . One animal experiment indicated that the effects of 2-week exposure of male mice to BPA can decrease sperm count and quality, and lead to the prevention of DNA damage in somatic and germ cells of mice [15] . De Flora et al. exposed male Sprague-Dawley rats to BPA for 10 consecutive days and found that BPA up regulated cluster in expression in atrophic prostate epithelial cells and induced lipid peroxidation and DNA fragmentation in spermatozoa [16] . However, to the best of our knowledge, seldom articles reported the effects of BPA on male sexual functions.
The present experiment investigated the effects of BPA exposure with different concentrationson the sexual behaviors of adult male mice. As far as we know, this research is the first time this topic was conducted on adult male animal mode. We selected 12‑week‑old male mice as an animal model for examining the effects of BPA on sexual functions and testosterone levels in vivo. In order to investigate whether the administration of BPA can have negative effects on the sexual behaviors of male mice, we tested several relevant items described above. The results indicated that BPA can prolong the latency of mount and intromissionbefore ejaculation, and decrease the frequency of them. No obvious difference of ejaculation latency in BPA-treated groups was seen compared with the control group. Copulatory efficacy was also declined by BPA. Accordingto previous articles, latency of mount and intromission isinversely proportional to sexual motivation, while ejaculation latency, intromission frequency, and copulatory efficacy are indicative of potency [17] [18] . That is, longer latency of mount and intromission implies lower sexual desire, while shorter ejaculate latency, lower intromission frequency and copulatory efficacy mean decreased potency. All of these results suggest that BPA may have a negative effect on sexual functions in male mice. Besides, according to our results, obvious dose-dependent effects were seen. The effect at the higher dose seemed more noticeable.
Previous studies have shown that the synthesis of testosterone is modulated by the HPG axis and is primary tothe maintenance of sexual behaviors [19] . Animal experiments indicated that the latencies to initial mount, intromission and ejaculation were significantly decreased in castrated male rats after castration [20] . In order to explore the mechanism of it, we also observed the effect of BPA on the testosterone synthesis in vivo. The results displayed that BPA can decrease the testosterone concentration in serum and testicular tissue, and the effects were more obvious in higher dose group than in lower dose group. As reported by previous articles, ITT is an absolute prerequisite for maintaining normal spermatogenesis [21] [22] . Normally, ITT concentration is roughly 50 - 100 times higher than serum testosterone concentration [22] . So, we suppose that BPA might impair Leydig cells, decline ITT, and then suppress spermatogenesis. But this hypothesis should be proved by further experiments.
In addition, the weights of reproductive organs were measured. We found that testis weight and relative testis weight decreased after the treatment of BPA. This phenomenon indicated that the function of testis might have been weakened. The weights and relative weights of epididymis and seminal vesicle also declined after the administration of BPA at the dosage of 100 mg/kg, and this might be attributed to the decrease of testosterone synthesis. These results also indicated that BPA might act in a dose-depen- dent manner.
5. Conclusion
In conclusion, BPA can supress the sexual behavior of adult male C57BL/6 mice, which might be attributed to the impairment of Leydig cells and the decrease of testosterone levels in vivo. So, reducing BPA in environment is very important to male health.
Acknowledgements
This work was funded by the Fundmental Research Funds for the Central Universities (16ykpy44).