Physiological Regulation of Valve-Opening Degree Enables Mussels Mytilus edulis to Overcome Starvation Periods by Reducing the Oxygen Uptake ()
Received 5 April 2016; accepted 30 May 2016; published 2 June 2016

1. Introduction
The filter-feeding blue mussel Mytilus edulis is a widely distributed and locally abundant bivalve mollusc in the North and Mid-Atlantic Regions, and a valuable commercial species [1] . The blue mussel is fully open and filters the ambient water at a maximum rate under optimal conditions, but at very low algal concentrations, the mussel reduces its valve-opening degree whereby the filtration rate becomes strongly reduced [2] - [6] . The valve closing-opening response to the absence and presence of algal cells has been thoroughly studied in M. edulis, and the critical algal concentration below which the mussel closes its valves is around 0.5 [4] to 0.9 µg chla L−1 [6] . The biological implications of this feeding behavior should be interpreted on the basis of naturally occurring phytoplankton biomass in the sea where the concentration is typically between about 1 and 5 µg chla L −1 [7] . But, because mussels are often living in dense beds, the actual phytoplankton biomass to which the mussels are exposed may frequently be strongly reduced [8] - [12] , and further, starvation often occurs in winter periods with no primary production [13] . During such starvation periods M. edulis reduces its valve gape, and thus the filtration rate, whereby the oxygen uptake becomes reduced because the oxygen uptake is governed by a diffusive boundary layer between the laminar ventilation current and the mantle cavity [14] - [16] . On this background it has been hypothesized that M. edulis may save energy by reduced metabolism during starvation periods by reducing the valve gape [17] , and recently this hypothesis has been experimentally tested by Riisgård and Larsen (2015) who measured the actual body-weight loss of mussels during a long-term starvation period, and subsequently compared the actual weight loss with the estimated body-weight loss assuming that the respiration rate was similar to that of fully open and filtering mussels [18] . Thus, it was found that the actual weight loss was 10 to 12 times lower than the estimated respiratory weight loss [18] , and this result supports the suggestion that physiological regulation of the valve-opening degree is an efficient mechanism that allows mussels to survive long periods of starvation.
In the present study we tested the hypothesis that Mytilus edulis during starvation regulates the opening degree of its valves in such a way that the oxygen concentration in the mantle cavity is reduced in order to minimize the respiration and at the same time prevent anaerobic metabolism which is energetically expensive [19] [20] . This was experimentally done by measuring the oxygen-concentration changes in the mantle cavity of both starved and fed mussels using a fibre-optic oxygen meter with a small sensor inserted into the mantle cavity through a hole drilled in the valve.
2. Materials and Methods
2.1. Collection of Mussels
Blue mussels (Mytilus edulis) were collected at the inlet to Kerteminde Fjord, Denmark, in February 2014 and kept in running seawater from the collecting site at the nearby Marine Biological Research Centre until the experiments were performed.
2.2. Measurement of Oxygen Concentration
2.2.1. Experimental Set-Up
The set-up used for measurement of oxygen concentration inside a mussel is shown in Figure 1. A 5.0 mm in diameter hole was drilled through the shell of a mussel, the mantle below was carefully cut open using a scalpel and a pair of tweezers. A plastic tube was inserted into the mantle cavity of the mussel. The end of the tube was sealed with an optic sensor spot, a fiber sensor was fixed close to the sensor spot within the tube and connected to an oxygen meter joined to a computer collecting the measured data. As seen in Figure 1 the optic sensor spot is located a short distance above the inner mantel surface, hence probably in the concentration boundary layer (see Discussion).
![]()
Figure 1. Mytilus edulis. Measurement of oxygen concentration inside a mussel. (Left) Fibre- optic oxygen meter connected to a sensor inserted into the mantle cavity of a mussel. The oxygen meter is connected to a computer collecting the measured data. (Right) Photo of an open and filtering mussel with a fiber sensor inserted into the mantle cavity.
2.2.2. Testing of the Set-Up
To test whether the set-up affected the normal feeding behavior of mussels, the filtration rate of 3 mussels was measured before and 14 d after a fiber optic sensor was inserted into the mantle cavity. The filtration rate was measured as the volume of water cleared of suspended particles that are 100% efficiently retained by the gills per unit of time. A mussel was placed in a 4-l experimental aquarium with well-mixed seawater added about 6 µm diameter algal cells (Rhodomonas salina) with an initial concentration of about 5000 cells ml−1. The reduction in the number of algae cells as a function of time was followed by taking water samples every 10 min and measuring the concentration with an electronic particle counter (Elzone 5380). The filtration rate (F) was determined from the exponential decrease in algal concentration (verified as a straight line in a semi-ln plot) as a function of time using the formula [5] :
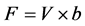 , (1)
, (1)
where V = volume of seawater in the experimental chamber, b = slope of regression line in a semi-ln plot for the reduction in algal concentration with time.
2.2.3. Measurement of Oxygen Concentration inside Mantle Cavity of Mussels
To investigate the effects of valve-opening degree on the oxygen concentration in the mantle cavity, the oxygen concentration both inside and outside the mantle cavity was measured simultaneously in 3 starved or fed mussels. The mussel was placed in a 4-l aquarium with bio-filtered seawater (20 psu) and the inserted fiber sensor then connected to the oxygen recording system. The oxygen concentration was measured every 10 or 30 s using a fibre-optic oxygen meter (Fire Sting O2, Pyro Science GmbH, Aachen, Germany) and a computer with Pyro Oxygen Logger software (Figure 1). After different periods of starvation, algal cells (Rhodomonas salina) with initial concentration of about 5000 cells ml−1 were added to stimulate the mussel to fully open its valves. During measurements, the valve-opening degree of the mussel was photographed using a digital camera (Olympus µTough-8010) at various time intervals.
2.2.4. Measurement of Respiration Rate
To investigate the effect of starvation on respiratory activity of mussels, the oxygen uptake of initially starved and subsequently fed mussels was measured. The respiration rate was measured as the consumption of oxygen in a closed respiration chamber (1.3 l) submerged in a temperature controlled water bath (17˚C) and the bio-filtered seawater within the chamber was well-mixed by means of a magnetic stirrer. The chamber was sealed and the respiration rate measured as the rate of oxygen consumption. The oxygen concentration inside and outside the mantle cavity of the mussel was measured simultaneously every 2 min. To measure the respiration rate on a fully open mussel, the mussel was fed algal cells (Rhodomonas salina) by adding an initial concentration of about 5000 cells ml−1. The oxygen concentration was plotted as a function of time, and expressed by a linear regression line. The respiration rate (mg O2 min−1) was calculated as:
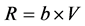 , (2)
, (2)
where b = slope of the regression line, V = water volume in respiration chamber.
2.3. Statistical Analysis
The differences in filtration and respiration rates between the starved and fed mussels were investigated using the Student’s t-test with a 5% significance level using SPSS 11.5 statistical software (SPSS Inc., Chicago, IL, USA).
3. Results
The filtration rates of 3 mussels before and after insertion of a fiber-optic sensor into the mantle cavity are shown in Table 1, and in Supplementary Data, Figure S1, and it is seen that filtration rates were nearly identical before and 14d after the operation, indicating that their feeding behavior was not affected.
Figure 2 shows two examples of measurements of simultaneous oxygen concentration inside and outside the
![]()
Table 1. Mytilus edulis. Mean (±SD, n = 3) filtration rates (l h−1) measured in 3 mussels before and 14 d after insertion of a fiber-optic sensor into the mantle cavity.
![]()
Figure 2. Mytilus edulis. Oxygen concentration measured simultaneously inside and outside the mantle cavity of a mussel (Mussel #1) kept in an aquarium during two series of measurements (a) & (b) where also the valve-opening degree of the mussel was photographed (c) & (d) at various time intervals. In both experiments the mussel was initially starved (and more or less closed), but after 385 min (a) and 218 min (b), respectively, algal cells (Rhodomonas salina) were added to the aquarium in order to stimulate the mussel to open its valves.
mantle cavity of a mussel. In the first example (Figure 2(a) and Figure 2(c)), the mussel gradually closed its valves over time when starved, but fully opened within 60 min after algal cells (Rhodomonas salina) were added to the aquarium (Figure 2(c)). Concurrently with the closing of valves, the oxygen concentration in the mantle cavity decreased until it reached zero where it remained until it rapidly increased after algal addition and subsequent valve opening (Figure 2(a)). In the second example, no oxygen was recorded inside the mantle cavity when the mussel was closed, but 20 min after addition of algal cells which stimulated the mussel to open its valves, the oxygen concentration rapidly increased to the nearly same level as outside (Figure 2(b) and Figure 2(d)).
Figure 3(a) shows that the oxygen concentration inside the mantle cavity of 2 fully open mussel fed algal cells was nearly constant and only somewhat lower than in the outside water of the aquarium (Figure 3(a)). However, during a subsequent starvation period the oxygen concentration in the mantle cavity showed pronounced variations (Figure 3(b) and Figure 3(c), Supplementary Data, Figure 2S). It is seen that the variation
![]()
Figure 3. Mytilus edulis. (a) Oxygen concentration measured simultaneously inside and outside the mantle cavity of 2 fully open mussels kept in an aquarium and fed algae cells (Rhodomonas salina). (b) Oxygen concentration inside the mantle cavity of 2 starved mussels. (c) Oxygen concentration inside the mantle cavity of same Mussel #2, again initially starved, but later on stimulated to increase its valve-opening degree by addition of algal cells to the aquarium (indicated by arrows).
in oxygen concentration is to some degree periodic. Thus, in Mussel #1 (Figure 3(b)) the oxygen concentration initially decreased from about 8 mg O2 L −1 (near the saturation level) to almost zero after 60 min, followed soon after by a rapid increase to 8 mg O2 L −1 in 10 min, and then again decreased to zero during the following 65 min. Due to valve closure, the oxygen concentration in the mantle cavity decreased to zero, but the duration of the this state did not exceed 7 h (Supplementary Data, Figure 2S). In general, the oxygen concentration in the mantle cavity of starved mussels was lower than outside, but when fed algae cells, the inside oxygen concentration increased rapidly to the level of the outside seawater (Figure 3(b) & Figure 3(c)).
Figure 4 shows the measured oxygen concentration inside and outside the mantle cavity of 3 mussels kept in a closed chamber, initially during starvation (Figure 4(a), Figure 4(c) and Figure 4(e)) and subsequently fed by adding algal cells to stimulate the mussels to fully open their valves (Figure 4(b), Figure 4(d) and Figure 4(f)). The measured respiration rates of the mussels are shown in Table 2 and it is seen that the respiration rate during starvation is about 40% of that measured in the fed mussels. During starvation, the oxygen concentration inside the mantle cavity fluctuated while declining to near zero (Figure 4(a) and Figure 4(e)). But when the mussels were stimulated to fully open their valves, the oxygen concentration recorded in the mantle cavity and outside seawater became almost identical and simultaneously decreasing (Figure 4(b), Figure 4(d) and Figure 4(f)).
![]()
Figure 4. Mytilus edulis. Decrease in oxygen concentration measured simultaneously in a closed respiration chamber and inside the mantle cavity of 3 mussels (#3, #4 and #5) kept in the chamber under initial starvation (left panel: (a), (c), (e)) followed by addition of algal cells to stimulate the mussel to open its valves (right panel: (b), (d), (f)).
![]()
Table 2. Mytilus edulis. Mean (±SD, n = 3) respiration rates (mg O2 h−1) of 3 mussels being starved and subsequently fed with algal cells.
Note: Within the same row, values with different superscripts are significantly different (P < 0.05).
4. Discussion
The present study supports the hypothesis that Mytilus edulis closes its valves during starvation in order to reduce the ventilation rate and thereby save energy by reducing the respiration rate. Thus, when there were no algal cells in the ambient water, the mussels gradually closed their valves (Figure 2(c) and Figure 2(d)) resulting in a decline of the filtration (ventilation) rate along with a simultaneous decrease in the oxygen concentration in the mantle cavity (Figure 2(a) and Figure 2(b), Figure 3(b)) and subsequently a remarkable decrease in the respiration rate (Table 2 and Figure 4). This suggests that the metabolism of mussels is down-regulated during starvation periods in order to save energy.
The present demonstration of valve closure in Mytilus edulis during starvation periods is in agreement with Eriksen and Iversen (1997) who observed that when there were no food particles in the ambient water the valves of M. edulis were only slightly opened, but after addition of algal cells the valves opened within 1 to 2 h and a 50% increase in respiration rate was recorded [21] . After incipient starvation Riisgård et al. (2003) also found that M. edulis closed its valves and that subsequent addition of algal cells stimulated the mussel to open so that maximum filtration rate was restored within 20 to 50 min [3] .
The change in respiration rate before and after addition of algal cells observed here is consistent with Famme (1980) who found that the oxygen consumption rate of unfed Mytilus edulis was about 57% of that of mussels fed algal cells, and further that the oxygen consumption rate of closing M. edulis momentarily became reduced to an at least 6 times lower level than that of “open” mussels [14] . Thompson and Bayne (1972) reported that the oxygen uptake of mussels after two weeks of starvation was less than 50% of that measured when the mussels had been fed [22] . Further, Taylor (1976) reported that the oxygen tension in the mantle cavity of the clam Arctica islandica decreased exponentially after valve closure [23] . According to Jørgensen et al. (1986), the water flow through the mantle cavity of M. edulis is laminar and the oxygen uptake determined by diffusion through boundary layers, and therefore, a reduction in respiration rate may be expected to be closely correlated with reduced valve gape and reduced water through-flow. When M. edulis partially closes its valves this interferes with normal irrigation of the mantle cavity and causes an increase in the thickness of the diffusive boundary layer implying that the oxygen uptake rapidly decreases [16] .
In the present study, starved mussels closed their valves for a certain period of time followed by a short period when they opened again which resulted in an alternating fall and rise of the oxygen concentration in the mantle cavity (Figures 2-4, Figure 2S). The pattern of the fall or rise of oxygen concentration within the mantle cavity varied greatly between individual mussels and between repeated measurements, but uniformity in tendency of the closing-opening pattern is evident. The valve movements of unfed M. edulis were measured continuously over several days by Famme and Kofoed [ [15] , Figure 1 therein] who recorded that the closed condition predominated, the times spent open being short, and that there was “a good deal of variation in both the degree of opening and in the time spent closed in a single organism” [15] . Ameyaw-Akumfiand Naylor (1987) also observed fluctuating valve-gaping in unfed M. edulis [24] . In 5 species of molluscs fed twice a week, Abele et al. (2010) measured the oxygen partial pressure (PO2) in the mantle cavity and observed a high intraspecificvariability of valve-opening behavior over 3 days, and they found that the frequency-distribution of measured PO2 values ranged from 0 to near normoxic 21 kPa, corresponding to about 8.9 mg O2 L −1 [25] . Further, Wilson et al. (2005) reported that M. edulis in a flow-through aquarium system showed similar basic gaping behaviour, but the gape angle varied either slowly over time or decreased rapidly over time followed by complete closure [26] . Thus, the starvation valve-gaping response is likely to be due to a certain requirement for oxygen. Although M. edulis can live anaerobically for several weeks with the valves completely closed, the mussel tends to use oxygen when available because that allows a more economical use of the stored fuel reserves, mainly glycogen [18] - [20] . The anaerobic respiration has a net yield of 2 ATP molecules per molecule glucose degraded, whereas the aerobic route yields 36 ATPs [19] . Although M. edulis can survive by anaerobic metabolism during hypoxic or anoxic conditions [27] , long-term anoxia is harmful to bivalves [28] [29] . Therefore, during starvation M. edulis frequently closes its valves to reduce the respiration to save energy, but because the mussel also needs oxygen to prevent anaerobic metabolism it subsequently opens the valves for a while to ventilate and take up oxygen.
In many previous studies, the oxygen consumption (metabolic rate) of Mytilus edulis has been classified by 3 functional states: standard, routine, and active [20] [22] [30] - [33] . The “standard rate” is the oxygen uptake during starvation, eventually reaching a steady-state value. Subsequently, when the starved mussels are fed and the rate of oxygen uptake rapidly increases to a high level this is termed the “active rate”. The respiration rate between the standard and active levels is designated the “routine rate”. However, from the present study it appears that the different respiration rates in M. edulis is simply a consequence of the varying valve-opening degree rather than a specific type of metabolism reflecting the energetic costs of water processing and feeding, as earlier pointed out by Jørgensen (1990) who found that the suggested classification of metabolic rates was unwarranted [17] . Thus it may alternatively be concluded that during a period of starvation M. edulis closes its valves, thereby reducing the oxygen uptake. After subsequent addition of algal cells, the mussel is stimulated to re-open to filter-feed and the oxygen uptake increases again, resulting in a higher respiration rate (Table 2, Figure 2). In this way, the low oxygen consumption rate of mussels in particle-depleted water can be interpreted as a physiologically regulated mechanism that allows mussels to save energy during starvation.
Considering the frequency with which mussels in the field experience shorter or longer periods with low algal concentrations [3] [4] [8] [9] [11] [12] [34] [35] , it is of great importance to understand the valve opening-closing mechanism and its bioenergetic implications [14] [16] - [18] . The present study shows that physiological regulation of the valve-opening degree is an efficient mechanism that allows mussels to deal with periods of starvation.
Acknowledgements
Thanks are due to Per Andrup for technical assistance and to Professor Poul S. Larsen for constructive comments on the manuscript.
Supplementary Data
![]()
Figure 1S. Mytilus edulis. Semi-ln plot of algal concentration (C, cells ml−1) as a function of time in filtration rate experiments with 3 mussels before (a) and 14 d after (b) insertion of a fiber- optic sensor into the mantle cavity. Each experiment was repeated 2 or 3 times by making new algal additions to re-establish the initial algal concentration. The estimated individual filtration rates using Equation (1) are shown in Table 1.
![]()
![]()
Figure 2S. Mytilus edulis. Oxygen concentration recorded simultaneously inside and outside the mantle cavity of 2 mussels during starvation. The oxygen concentrations were measured every 10 s using fibre-optic sensors.
NOTES
![]()
*Corresponding author.