Nomogram of Follicle Stimulating Hormone Starting Dose Based on Antral Follicle Count, Third Day Follicle Stimulating Hormone Levels, and Age in Hasan Sadikin Hospital Bandung ()
Received 22 February 2016; accepted 23 May 2016; published 26 May 2016

1. Introduction
The main aspect of IVF cycle individualization is to offer every patient the best therapy adjusted to each individual characteristic. Therefore, allowing the possibility of a high success rate, and also minimalizing risks that originate from ovarian stimulation. Choosing a different gonadotropin dosage for each patient is a significant clinical decision in personalizing therapy. Although exogenous FSH has been used for decades, and millions of cycles have been performed all over the world, the criteria to choose the correct initial dose of FSH have not yet been identified. Usually, clinicians choose the FSH starting dose based on anamnesis and clinical criteria. The most important is the results of previous IVF. If there are no previous cycles, the criteria will be based on the patient’s age, BMI, and markers of ovarian reserve [1] [2] .
One of the markers currently used to determine ovarian reserve is AFC, which has one of the best performances in predicting ovarian response towards exogenous FSH [3] - [5] . Ovarian antral follicle with a size larger than 2 mm is very sensitive and responsive towards FSH and is defined as recruitable. They can be seen and measured with transvaginal ultrasonography (USG). AFC is the amount of follicles sized 2 - 10 mm from both ovarium [6] [7] . Therefore, AFC can predict with high accuracy how well the follicle pool responds to exogenous FSH.
There are published studies reporting the excellent correlation between AFC and ovarian response in the IVF program [8] [9] . AFC can also correlate with the event of menopause transition that indicates a close relationship with the quantitative aspects of ovarian reserve [10] . The advantage of AFC is the possibility that the measurement is performed concurrently while the clinician examines the patient, therefore there is possibility that AFC will always be the marker widely used to determine ovarium reserve.
Recently, a study published a nomogram to calculate the FSH starting dose in the IVF cycle [11] - [13] . The nomogram is based on patient’s age, third day FSH levels, and anti-mullerian hormone (AMH), and also can be a reference for individualized dosage for all patients in the healthcare setting that uses AMH to determine ovarium reserve. Meanwhile, the important role of AFC in identifying extreme ovarian response has been proved [8] [9] . Its role in therapy individualization such as FSH starting dose needs to be further explained. Therefore, we intend to investigate whether it is possible to create a nomogram based on AFC, so clinicians can decide on the correct initial exogenous gonodatropin dose in the IVF cycle, whose purpose is to reduce the possibility of extreme ovarian response.
The issue is whether an FSH starting dose nomogram based on AFC amount, third day FSH serum levels, and age are suitable to female patients who are going through IVF in Aster Clinic Hasan Sadikin Hospital Bandung. The purpose of this research is to create a nomogram based on AFC and age to calculate the correct FSH starting dose for IVF therapy in Aster Clinic Hasan Sadikin Hospital Bandung.
2. Methods
The study design was retrospective cohort. The study was held in March to May 2015 at Aster Clinic Hasan Sadikin Hospital Bandung. The study was done on patients going through IVF therapy at Aster Clinic Hasan Sadiking Hospital Bandung. In this study the sample size was selected for the study purpose was 60 people. This research has been approved by ethics committee in Faculty of Medicine, Universitas Padjadjaran.
The research type in this study was observational analytic with a retrospective cohort design, which is a type of research that study outcomes which are evaluated at certain time points. This type of research seeks to study the dynamic of relationships or correlations between risk factors with its impact or effects. Risk factors and impacts or effects was observed simultaneously, which means that the research subject was observed once and risk factors including effects was measured based on the situation or status at the time of observation. Stages of this research can be seen in Figure 1.
3. Data Analysis
The obtained data were recorded in the research form which was previously created, then was edited, verified,
coding and data entry, and afterwards the data were analyzed. Analysis was performed to obtain the prevalence value, which was retrospectively analyzed data from 60 medical records that contain clinical information and AFC of patients that successfully participated in the IVF program and data demographics of research subjects at the Aster Clinic dr. Hasan Sadikin General Hospital.
Appropriate statistical analysis of research objectives and hypotheses is used to determine the correlation between them. Statistical analysis for categorical data was tested by Chi-Square test and Fisher test and Kolmogorov-Smirnov test as the alternative if the requirement of the Chi-Square is not met. Meanwhile, to test the hypothesis of statistical correlation between the two numeric variables is using Pearson correlation test if the data were normally distributed and Spearman correlation test as the alternate if the data are not normally distributed. To analyze the magnitude of correlation is using criteria Guilford. Interpretation the results of hypothesis testing based on the strength of the correlation, the direction of correlation, and p-value: The strength of correlation (r) based on criteria Guillford (1956), The data obtained are recorded in a special form and then processed through SPSS version 21.0 for Windows.
In this discussion which is simple linear regression analysis, the linear relationship observed is a linear relationship between the independent variables with one variable dependent.
4. Results and Discussion
The purpose of the study is to determine the dose of FSH by the value of AFS and age of patients, carried out using multiple linear regression analysis. Before performed a multiple regression analysis, there are some sort of test:
4.1. Classical Assumption Test
4.1.1. Normality Test
One of the assumptions that must be fulfilled in the establishment of best regression model (BLUE) is a normal distribution of the residual is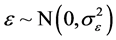 , which means that the data analyzed samples taken from normally distributed population. In this study, the normality test was done descriptively by looking at the graph plots between the value of the dependent variable and its residual. Normality in the regression model can be seen from Normal P-P Plot of output SPSS 21.0 as seen in Figure 2.
, which means that the data analyzed samples taken from normally distributed population. In this study, the normality test was done descriptively by looking at the graph plots between the value of the dependent variable and its residual. Normality in the regression model can be seen from Normal P-P Plot of output SPSS 21.0 as seen in Figure 2.
Figure 1 shows clearly that the data points tend to follow a straight line so that it can be concluded that the error (ε) is in normal distribution. This indicates that the regression model to meet the standard distribution of normality so that the normality assumption is fulfilled.
4.1.2. Test Multicollinearity
After normality test, further multikolenieritas assumptions test needs to be done. A good regression model is a regression model that is free of the multicollinearity between independent variables. Multicolinierity test is done by looking at the tolerance value and Variance Inflation Factor (VIF). Multicollinearity happened if VIF above of 10 or the tolerance value below 0.10. If the VIF for the independent variable is greater than 10, then one variable that correlated among variables should be reduced from the regression model. Multicollinearity is a very high correlation or very low correlation that occurred between the relationship of the independent variables. Multicollinearity test in this study is done by using Variance Inflation Factor (VIF) and output obtained by SPSS 21.0 as follows:
From the multicollinearity test results in Table 1, VIF is smaller than the 10 or tolerance above 0.1. From the table was obtained that all VIF values below 10 or tolerance values above 0.1, so that it can be concluded there are no multicollinearity between independent variables that exist. It can be concluded that there are no symptoms of multicollinearity between independent variables. Thus, the regression equation meet the assumptions of non-multicollinearity.
4.1.3. Heteroscedasticity Test
Heteroscedasticity indicates that the variance of variable is not the same for all observations. Heteroscedasticity test using scatterplot test. The purpose of heteroscedasticity test is to examine whether the regression model occurred inequality variance of the residuals of an observation to another observation. A good regression model means there is no heteroscedasticity. To detect the presence of heteroscedasticity is done by embodying a scatter plot between the estimated value with the residue. The heteroscedasticity test results are shown in Figure 3:
As seen in Figure 3, the points spread randomly between the zero axis of the bottom and top, and do not form a particular pattern. It can be concluded that there is no heteroscedasticity in research. So the asumption of heteroscedasticity is fullfilled.
4.2. Correlation Analysis
To determine the relationship between the variables in the study, the variables in this research are the value of AFS and age of patients with variable dose FSH. Calculations were performed using SPSS 21.0, with the following results:
The results from Table 2, to interpret the correlation used the following criteria:
1) 0 to 0.19: correlation is very low.
![]()
Table 1. Multicollinearity test results.
![]()
![]()
Table 2.Spearman correlation test results.
**. Correlation is significant at the 0.01 level (2-tailed).
**. Correlation is significant at the 0.01 level (2-tailed).
2) >0.20 to 0.399: low correlation.
3) >0.40 to 0.599: correlation is strong enough.
4) >0.60 to 0.799: strong correlation.
5) >0.80 to 1.000: very strong correlation.
So we can conclude that the correlation between the dose of FSH and the age of the patient are included in the low category, while the correlation between the dose of FSH and AFC are included in the strong enough category.
4.3. Determination Coefficient
To see the magnitude of linear relationship between age of the patient and the dose of the AFC and FSH, it can be based on the calculation of SPSS 21.0 statistical software by looking at the numbers R Square as below.
From Table 3, it can be seen that the results of R Square is 0.281 which shows the influence of independent variable (X) is the age of the patient and the AFS to FSH (Y). The amount of Coefficient Determination (CD) can be formulated as follows:
KD = R Square × 100%.
![]()
Figure 3. Heteroscedasticity test results.
a. Predictors: (Constant), AFC AMOUNT, PATIENT AGE; b. Dependent Variable: FSH DOSE.
KD = 0.281 × 100%.
KD = 28.1%.
This figure shows the results of determination coefficient of independent variable (X) and explain it’s influence or relationship to the dose of FSH (28.1%). While the remaining 71.9% influenced by the other factors that are not examined.
4.4. Hypothesis Test
Hypothesis test in this study is divided into two, the simultaneous test using the F test and partial test using T test.
a. Hypothesis Test Simultaneously
Hypothesis test simulataneously by F test. If p value less that 0.05 (p < 0.05) means it’s statistically signi- ficant. The result of hypothesis test in this study are shown in Table 3 and Table 4.
From the result of hypothesis test simultaneously obtains the number of significance 0.000. So H0 is rejected and H1 is approved. The number of result means that the alternative hypothesis IH1 is acceptable because 0.000 < 0.05 and there is a linear relationship between age of patients and AFC to FSH.
![]()
Table 4. Hypothesis test simultaneously (F Test).
a. Dependent Variable: DOSIS FSH; b. Predictors: (Constant), AFC Total, Age.
4.5. Partial Hypothesis Testing
We used T test as partial hypothesis testing and analyze its number of significance number. If P < 0.05, H0 is rejected and H1 is accepted. To measure the size of significance we used Beta of standardized coefficients. If it is <0.05, we can conclude that hypothesis is accepted. The result of hypothesis testing is showed in Table 5.
Out of 60 subjects with 5% significant level, we concluded that not all variable has significant result.
4.6. Multiple Linier Regression Analysis
Multiple linier regression analysis is used to predict dependent variable based on its independent variable. In this study, independent variables (X) were patient’s age and AFC level, which used to predict dosage of FSH. The formula of linear regression analysis used in this study:
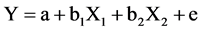
Note:
Y: FSH dose.
X1: Patient’s age.
X2: AFC level.
a: Constant.
b1, b2: Regression coefficient.
e: error term.
Here is the result of multiple linear regression analysis from statistic software of SPSS 21.0:
Result of significance test in Table 6 showed P = 0.000. p value used in this study is <0.05, thus in this study H0 was rejected and H1 was accepted. From Table 6 we can conclude that patient’s age and AFC level is signi- ficant simultaneously and they were significant to FSH dose. The linear regression result and spearman correlation analysis results are shown in Table 7 and Table 8 respectively.
From Table 7, the result of multiple linear regression model was included into the regression formula. Here is the formula in this study:
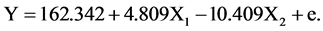
4.7. Regression Analysis of Basal FSH and FSH Dose
4.7.1. Correlation Analysis
We conducted correlation analysis to mesure significance of correlation between all variable in this study. Vari- ables are AFS level and patient’s age with FSH dose variable. Analysis was done using SPSS 21.0, as follows:
We used criteria below to interpret the result:
1) 0 - 0.19: very low correlation.
2) >0.20 - 0.399: low correlation.
3) >0.40 - 0.599: moderately strong correlation.
4) >0.60 - 0.799: strong correlation.
5) >0.80 - 1.000: very strong correlation.
Therefore we concluded that FSH dose and Basal FSH had low correlation.
![]()
Table 5. Partial hypothesis analysis.
a. Dependent Variable: FSH DOSE.
![]()
Table 6. Result of multiple linear regression analysis (ANOVA).
a. Dependent Variable: FSH DOSE; b. Predictors: (Constant), AFC LEVEL, PATIENTS AGE.
![]()
Table 7. Result of multiple linear regression (coefficient).
a. Dependent Variable: FSH DOSE.
![]()
Table 8. Result of spearman’s correlation testing.
**. Correlation is significant at the 0.01 level (2-tailed).
4.7.2. Coefficient of Determination
To see the linier correlation between basal FSH and FSH dose, we used R square in SPSS 21.0, as follow:
From Table 9, we conclude that R Square = 0.281. This showed significance of independent variable X, basal
a. Predictors: (Constant), BASAL FSH; b. Dependent Variable: FSH DOSE.
FSH, to FSH dose (Y). The value of coefficient of determination coefficient (CD) is formulated as:
CD = R Square × 100%.
CD = 0.067 × 100%.
CD = 6.7%.
The result showed CD of independent variable (X) and explained its correlation or association with FSH dose as 6.7%. While the rest, 93.3% is affected by other unidentified factors.
4.8. Hypothesis Testing
Hypothesis testing in this study was a partial testing using T test.
4.8.1. Partial Hypothesis Testing
Partial hypothesis testing using T test was conducted to analyse the number of significance. If P < 0.05, we reject H0 and accept H1. To measure the size of significance, we used Beta of Standardized Coefficient. If P is <0.05, we accept alternative hypothesis. The result of partial hypothesis testing in this study stated in this Table 10.
Out of 60 patients, with 5% level of significance, from partial hypothesis testing, we conclude that basal FSH has no significant correlation with FSH dose.
4.8.2. Simple Linear Regression Analysis
Simple linear regression analysis is used to to predict dependent variable based on its independent variable. In this study, dependent variable (X) was basal FSH, which used to predict FSH dose. The formula used in this study was:
![]()
Notes:
Y: FSH Dose.
X1: Basal FSH.
a: Constant.
b1: Coefficient Regression.
e: error term.
Below is the result of simple linear regregession analysis in SPSS 21.0 software:
Result of significance testing of Table 11 shows that P (Sig.) was 0.045. Level of significance use in this study is 0.05, thus we rejected H0 and accepted H1. Therefore from Table 11, we can conclude that Basal FSH was statistically significant to FSH dose. The regression formula is shown in Table 12.
From Table 12 in a simple linear regression model, we input the number into a regression formula. Here is the formula in this study:
![]()
From the results of a statistical analysis of multiple parameters, we made a nomogram based on age and level of AFC (Figure 4) and based on the levels of FSH (Figure 5). In Figure 4, we can predict the starting dose of FSH based on age and AFC more easily. While in Figure 5, we can predict not only starting dose, but also basal FSH level.
5. Conclusion
Conclusions of this study are some variables such as age and the AFC can be used as a basis for making nomo-
a. Dependent Variable: FSH DOSE.
![]()
Table 11. Result of simple linear regression analysis.
a. Dependent Variable: FSH DOSE; b. Predictors: (Constant), BASAL FSH.
![]()
Table 12. Result of simple linear regression analysis (coefficient).
a. Dependent Variable: FSH DOSE.
![]()
Figure 4. Nomogram of age and AFC level.
gram to calculate the correct FSH starting dose for IVF therapy. Its validity and accuracy still need to be confirmed.