Synthesis of SiOx Nano-Powders Using a Microwave Plasma Torch at Atmospheric Pressure ()
Received 16 February 2016; accepted 26 April 2016; published 29 April 2016

1. Introduction
Nanostructures have attracted considerable attention in many research fields due to their unique low-dimensional quantum size effects, interesting geometry, and potential applications in nanotechnology. Silicon oxide nano- powders (SiOx-NPs) in particular have generated a great deal of interest for their outstanding semiconducting, mechanical, and optical properties, all of which enable them to be used as coating materials, sensitive sensors, protective layers, and blue-light-emitting diodes. These future applications, however, impose more challenging standards for a range of cell perspectives, including energy density, power performance, cycle life, and safety. While issues in these parameters need to be addressed in parallel, the simultaneous improvement of the energy density and cycle life is more critical [1] - [3] . Of late, there has been a focus on SiOx-NPs (x ≈ 1) due its high charge/discharge efficiency and capacity as an alternative material to carbonaceous materials among secondary cell anode materials [4] - [7] . SiOx-NPs are usually synthesized using chemical reaction routes which are often complex and offer a relatively small production yield [8] [9] . The adsorptive capabilities of nano-scaled SiOx will exceed those of conventional SiOx. In this context, we report a simple synthetic method of preparing SiOx-NPs, making use of an atmospheric microwave plasma torch.
2. Experiment
Figure 1 shows the experimental setup for the preparation of SiOx-NPs with the microwave plasma torch. The design and operation of the atmospheric microwave plasma torch are briefly summarized here for completeness, although they have been reported in detail in our previous literatures [10] - [13] . As shown in Figure 1, the microwave energy is supplied to the flowing gas at atmospheric pressure by a microwave generator, and efficient power transfer is achieved through a matching network, which basically consists of an auto-matcher, a matcher controller, and an isolator, namely, the microwave radiation generated from the magnetron passes through the circulator and the auto-matcher, is guided through a tapered waveguide, and enters the discharge tube made of a fused quartz. The center axis of the quartz dielectric tube, with an outer diameter of approximately 30 mm and a thickness of 2 mm, is located one-quarter wavelength from the short end of the waveguide and is perpendicular to the wide waveguide walls. The electric field induced by the microwave radiation in the quartz tube can be maximized by adjusting the auto-matcher. Additionally, the reflected power adjusted with the auto-matcher is less than 1% of the forward power. This produces a plasma torch with a high temperature and a high plasma density. The microwave plasma torch provides a highly unusual and reactive chemical environment at high temperatures. For example, the air microwave plasma torch produces plasma with a high temperature of ~6000 K and a high plasma density of ~1013/cm3 [14] .
All the gas flows in Figure 1 were controlled by mass flow controllers (MFCs). The silicon source in our experiment was silicon tetrachloride (SiCl4), which has a high vapor pressure, low activation energy, and low cost.
![]()
Figure 1. The presentation of the synthetic system of SiOx-NPs with the atmospheric microwave plasma torch.
The properties mentioned above may be suitable for a plasma-enhanced gas-phase synthesis method at atmospheric pressure. SiCl4 is handled in a glove box with a non-moisture atmosphere, and a bottle for SiCl4 bubbling has to be packed well because of a moisture-sensitive reagent. SiCl4 (99.9%, Aldrich) in the liquid solution is directly bubbled by argon gas and is axially injected with hydrogen gas through the Teflon tube, as shown in Figure 1, which guides a mixture of bubbled SiCl4 and hydrogen gas into the center of the plasma flame. As shown in Figure 1, SiCl4 in liquid solution and the inlet line of axial gases were maintained at about 65˚C by making use of automatic-controlled heaters. Nitrogen and air as swirl gas enter the microwave plasma torch via four small holes in the tangential direction of the inner surface of the quartz tube, not shown in Figure 1, and hydrogen as an additive gas was used for partial oxidation of silicon oxide formed from the decomposition and reduction of SiCl4. The SiOx-NPs were synthesized by 20 lpm of N2 and 0.5 lpm air as swirl gases and 10 lpm of N2 gas with 10 lpm of H2 gas for SiCl4 carrier and then SiCl4 liquid pre-cursor of approximately 1 ml per minute (ml/min) were injected into the microwave plasma torch operated at 2 kW power. Furthermore, the swirl gases of N2 were injected before sampling to cool down because as-produced SiOx-NPs had avoided oxidation under high temperature conditions. Once the synthesis of SiOx-NPs starts by passing through the plasma flame, a red-brownish light, like a flash, emits from the torch flame, not shown in this paper. All the samples were taken from deposits inside the quartz tube.
3. Results and Discussion
Figure 2 depicts the graph of the size distribution bars with an inert scanning electron micro-scope (SEM) image, and energy dispersive spectrometer (EDS) spectra of synthesized SiOx-NPs. The photo image of Figure 2(a) is the synthesized SiOx-NPs by the microwave plasma torch. Kim et al. reported different colors of SiOx powders in terms of oxide x values. The SiOx sample colors were strongly dependent on their valence states and became brighter when x increased. When the x value was 1.18, the color was brown, whereas it was almost white at an x value of 1.83 [15] . Additionally, the size distribution of synthesized SiOx-NPs was analyzed using an SEM image (insert image) and the average size was approximately 230 nm, as shown in Figure 2(b). Figure 2(c) illustrates the EDS analysis data in the form of spectra, where the peaks corresponding to the elements Si, O, and carbon appear to be dominant, as seen in the spectra, and the data listed in Table 1 show a small amount of carbon. It is believed that the trace of carbon in the spectrum was detected due to the atmospheric pressure synthesis process and during the sampling of SiOx-NPs. For this reason, the carbon spectra were detected by EDS analysis. Hence, the synthesized SiOx-NPs were examined by performing an EDS analysis. To determine the valence state of the Si in the SiOx in synthesized SiOx, an X-ray Photoelectron Spectroscopy (XPS) analysis was conducted.
Figure 3(a) shows the Si-2p binging energy spectra of Si in the SiOx from the X-ray photoelectron spectroscopy (XPS). The analysis condition of XPS (AXIS Ultra DLD, Kratos Inc.) was a monochromatic Al Kα (1486.6 eV, 150W). The measured band of the Si-2p binding energy was divided into five sub bands: Si (99.8 eV), SiO0.5 (100.7 eV), SiO1.0 (101.5 eV), SiO1.5 (102.5 eV), and SiO2.0 (103.5 eV). The x value was calculated using the following equation [16] :
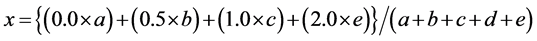
where, x is the valence state of the Si in the SiOx, and a, b, c, d, and e represent the intensity of the Si-2p binding of the Si, SiO0.5, SiO1.0, SiO1.5, and SiO2.0, respectively. The normalized intensities for calculating the x values are 0.53 of Si, 0.12 of SiO0.5, 0.37 of SiO1.0, 0.77 of SiO1.5, and 0.32 of SiO2.0, respectively. In this context, the x value of synthesized SiOx was 0.91. Additionally, a comparison with the X-ray diffraction (XRD) pattern of synthesized SiOx and commercial SiO2 NPs are shown in Figure 3(b). The commercial SiO2 NPs were prepared
![]()
Table 1. Spectrum data from EDS analysis of synthesized SiOx-NPs.
![]()
Figure 2. (a) Photo, (b) size distribution bars with insert SEM image, and (c) spectra data from EDS of synthesized SiOx-NPs.
![]() (a) (b)
(a) (b)
Figure 3. (a) The Si-2p binding energy spectra of synthesized SiOx-NPs from XPS (the value indicates a valence state of Si in SiOx) and (b) XRD spectra of synthesized SiOx and Ref. SiO2 NPs.
with over 99.5% of purity and 10 - 20 nm of average size from Sigma Aldrich (CAS No. 7631-86-9). The XRD pattern of synthesized SiOx-NPs is shown in Figure 3(b), red line, the Si (111, 220) diffraction peak. Further, Sun, W. et al., the commercial SiO precursor, has only two broad humps centered at ~23 and ~51 degrees and was observed in the XRD pattern [17] . In this context, the characteristics of synthesized SiOx-NPs which have a 0.91 x value are very close to the SiO.
Figure 4 shows the volumetric charge (delithiation) capacity of the anodes using the Ref. Si and synthesized SiOx-NPs as a function of cycle number and Coulomb efficiency at a 0.2 C rate between 0.01 and 1.5 V in a coin-type half-cell. The Ref. Si-NPs (average size of approximately 200 nm) showed before and after 100 cycles of charge/discharge capacity at 2343 mAh/g and 511 mAh/g, respectively. The Ref. Si-NPs showed that the highest first charge/discharge capacity faded out quickly with the increase in the number of cycles. Therefore, the retention
![]()
Figure 4. Cycle performances of the anodes using by the Ref. Si and synthesized SiOx-NPs.
ratio of Ref. Si-NPs is only 21.8%. However, the synthesized SiOx-NPs demonstrated very stable cycle performance. The synthesized SiOx-NPs showed before and after 100 cycles charge/discharge capacity at 1264 mAh/g and 1127 mAh/g, respectively. Therefore, the retention rate is 89.2% after 100 charge/discharge cycles. These results were due to the large portion of Li-based oxides such as Li2O and Li4SiO4 on the nano-particles. Li2O and Li4SiO4 are inactive to an electrical field, but they can serve as a buffer to the expansion of Si during the lithiation process. These buffers can result in good cycle performance from the next cycle [15] [18] [19] .
4. Conclusion
The microwave plasma torch method has been developed to synthesize SiOx-NPs. The high-temperature microwave plasma flame evaporated the SiCl4 pre-cursor and produced SiOx-NPs through the cooling of Si atoms in the downstream direction of nitrogen and air plasma torch. The SiOx-NPs can easily be obtained by making use of the microwave plasma torch, although the synthetic approach reported here is not finely controlled. The x value and average size of the synthesized SiOx were 0.91 and approximately 230 nm, respectively. The volumetric charge capacity is 1127 mAh/g and 89.2% retention after 100 cycles. This method may be suitable for direct continuous preparation and mass production of SiOx-NPs by adding a collection chamber, such as filtration apparatus. This work might provide the synthesis for controlling the x-value in SiOx-NPs by oxygen content.
Acknowledgements
This study was supported by R&D Convergence Program funded by Korea Research Council of Fundamental Science & Technology. This study was also funded by a grant in the National Agenda Project of the Korea Research Council of Fundamental Science and Technology.
NOTES

*Corresponding authors.