Effect of Soil Properties on Tree Distribution across an Agricultural Landscape on a Tropical Mountain, Tanzania ()
Received 17 February 2016; accepted 24 April 2016; published 27 April 2016

1. Introduction
Trees on farm provide multiple benefits of ecosystem services including food security that support human livelihood. They can further offer synergy between adaptation and mitigation in addressing climate change impacts [1] - [3] . Integration of trees on farming landscapes forms part of traditional farming systems in the tropics [4] , with varying degrees of practices [5] . Recently, there has been a noticeable trend of increasing tree cover on farms which coincides with diminishing forest cover in the natural forests due to overexploitation and deforestation [6] - [9] . Increased tree cover on farm had got upper hand as smallholder farmers actively engaged in priority setting and selection process of desired tree species [10] [11] .
The composition and structure of sustained tree-based landscapes can be shaped by environmental variables, institutional settings and socio-economic dynamics [12] [13] . The suitability of tree species communities depends mainly on environmental variables as demonstrated by ecological niche modelling [14] . However, the use of habitat suitability models has been limited due to deficiencies in regional datasets, poor predictive performances and inconsistencies in model projection using future climate conditions [15] . Alternatively, a combination of the niche modelling and ordination has been used to utilize the synergies of the two methods (e.g., [16] ), while others have proposed multiple parallel ordination methods [17] . The choice of environmental variables greatly influences the outcome of the ordination and must include key environmental variables used including climate (temperature and precipitation), topography (elevation and slope) and soil properties [18] [19] .
Most assessments on tree distributions using ordination methods have been conducted on natural forests and rangelands where natural disturbances and processes and environmental variables are key factors [20] [21] . This study attempts to improve the knowledge gap by inclusion of farmland in understanding influence of soil properties on tree species distribution. Specifically, the study attempted to, 1) characterize tree species diversity and their local use, and 2) determine distribution of tree species communities as influenced by soil properties.
2. Materials and Methods
2.1. Study Site
The study was conducted in Moshi district, which is among administrative districts within Kilimanjaro region in north-eastern Tanzania on the southern part of Mount Kilimanjaro (Figure 1). The study site is comprised of agricultural landscape that occupies the lower altitude of the Mount Kilimanjaro; below 1800 m a.s.l. and is categorized into three zones namely, upland (1800 - 1400 m a.s.l.), the midland (1400 - 900 m a.s.l.) and lowland (below 900 m a.s.l.), differentiated by topography, climatic conditions and associated land uses [22] [23] . The upland zone is characterized by precipitation range of 1250 - 2000 mm per annum and mean annual temperature of 24˚C; the midland zone receives precipitation ranges between 1000 and 1200 mm per annum and mean annual temperature of 26˚C; and, the lowland zone has annual mean temperature of 33˚C and rainfall ranges between 400 and 900 mm per annum [22] .
Mount Kilimanjaro is a stratovolcano made up of three main centres of Kibo, Mawenzi and Shira [24] . Soils are mover diverse especially in the midland (Haplic Phaeozom) and upland (Humic Nitisol), as a result of processes involving the volcanic ash [25] , while in the lowland savannah plain it is classified as Eutric Fluvisol [26] [27] . Hydrological processes of the study area is very complex, comprised of heavy precipitation, snow and deep ground water infiltration from the higher altitudes, and about 96% of the water inflow originate from the forest belt [28] [29] .
2.2. Methods
2.2.1. Tree Stocks Assessment
On-farm tree inventory was conducted using 1 ha square plot (100 m × 100 m, Figure 2), laid along transect from around 1690 to 680 m a.s.l. A total of 50 plots were established, and distributed by 12 plots in the upland,
![]()
Figure 1. Location of the study site on the slopes of Mount Kilimanjaro, Tanzania.
![]()
Figure 2. Plot layout for the tree inventory with nested four subplots (1 - 4) used to collection of the soil samples.
14 plots in the midland and 24 plots in the lowland (however, two plots in the lowland were treeless hence removed from further analysis). In each plot all trees ≥ 5 cm diameter at breast height (DBH) were identified by use of botanist, their uses described by farmers within farm plots and recorded in the field note book (coffee shrubs and lianas were excluded).
The vegetation types of the recorded tree species were classified according to elevation using guideline produced by NAFORMA [30] . Tree species diversity was computed using distribution indices by expressions (1), (2) and (3);
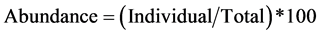 (1)
(1)
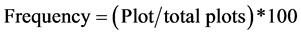 (2)
(2)
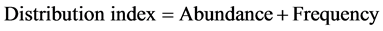 . (3)
. (3)
2.2.2. Soil Properties Assessment
Soil sampling plots were nested in the vegetation plots (Figure 2), using an inverted Y-shaped design adapted from AfSIS protocol [31] . Soil samples were collected at top (0 - 20 cm) and subsoils (21 - 50 cm) depth, at the four locations using the Y-shape for cumulative and composite samples. Cumulative soil sample was collected once at the centre (subplot 1) of each plot using an Edelman combination auger. A sampling plate was used as an auger guide to enable full recovery of the samples. Cumulative soil mass collected from top and subsoils were separately packed and labelled in zip-lock bags. Composite sample was collected in the four subplots (1 - 4), at top and subsoils, thoroughly mixed, and an estimated 700 g each from top and subsoils were packed separately in zip-locks and labelled.
All samples (cumulative and composite) were air dried in a large room and then weighed using calibrated top-pan balance to the nearest 0.1 g. Oven-dried subsamples were ground using wooden rolling pin, then sieved through 2 mm mesh, and the remaining course fragments > 2 mm weighed. About 50 - 150 g of the cumulative samples were oven-dried at 105˚C for approximately 48 hours until reached a constant weight. Gravimetric soil moisture content (MC) and bulky density (BD) were then computed using expressions (4) and (5);
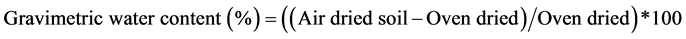 (4)
(4)
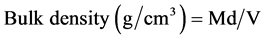 (5)
(5)
where; Md = mass of dry soil sample, V = soil volume
Air dried sub-samples from composite soil samples from top and subsoils each with approximately 20 g were loaded in to four wells and scanned using Fourier Transformation Mid Infrared Reflectance Spectroscopy at World Agroforestry Centre (ICRAF) Soil-Plant Spectral Diagnostics Laboratory in Nairobi. The soil samples were scanned 32 times and their four spectra averaged to account for variability within-sample and particle size and packaging in wells [32] . A sub-set of 30% of soil samples was randomly selected for wet chemistry analysis. These data were used for site-specific calibration and validation of the spectral predictions. Chemometric methods were used to predict soil properties from soil spectra and measured values of the reference soil samples [33] - [35] . The regression model developed from calibration was used to predict the soil properties for the rest of the samples and their coefficient of correlation (R2) and root mean standard errors of calibration (RMSEC) are shown in Table 1.
2.2.3. Statistical Analysis
Detrended correspondence analysis (DCA) was conducted to determine species spread and individual positioning in the landscape, and their correspondences with soil properties [36] . Soil properties that were found to be significant (p < 0.05) were considered for final DCA (Table 2, Figure 4). Additional analysis was done using
![]()
Table 1. Calibration results for soil properties on the slopes of Mount Kilimanjaro.
![]()
Table 2. Soil parameters and their corresponding DCA values on distribution of tree species on the slopes of Mount Kilimanjaro, Tanzania.
Note: Eigenvalues for DCA-1 axis = 0.677 and DCA-2 axis = 0.36.
Sorenson’s test to compare species spread among different land use zones. Species cumulative curves were drawn to show species increment with sampling sites, as an indicative measure of species diversity [37] . A normality test was conducted and indicated that all tree species distribution did not conform to normal tendency, hence non-parametric Kruskal Wallis (K-W) and Mann Whitney Wilcoxon (MWW) were used for statistical tests.
3. Results
3.1. Species Diversity and Local Use
A total of 69 species belonging to 28 families were recorded in the study site, comprised of multi-purpose tree species mainly catering for fruits, timber, fuelwood, and medicinal values (Table 3). At local scale, distribution index indicated that 77% of the species were rare, while 10% and 13% were occasional and abundant respectively. Species were found to overlap between land use zonesby more than 30% as indicated by Sorenson’s index (s = 0.33 - 0.38).
Detrended correspondence analysis (Figure 3(a)) indicated species spread in the lowland and clustering in the upland and the midland areas. Lowland plant communities have shown positive loadings along Axis-1, whilst upland plant communities showed negative loadings. Species were found to overlap between altitudinal zones by more than 30% as indicated by Sorenson’s index (s = 0.33 - 0.38). Species cumulative curves (Figure 3(b)) indicated increment of tree species with increased sampling sites. Rate of species accumulation differed between the three land use zones and was steepest in the midland, and shallower in the lowland.
Species richness (Figure 4(a)) exhibited a humped-shaped curve with altitude, increasing from the lowland up to the midland, and thereafter declining towards the upland, though the patterns did not show statistical differences (K-W: df = 40, p = 0.294). Using land use zones the same trend was observed (Figure 4(b)), with species richness highest in the midland followed by the upland, and lowest in the lowland. The differences were significant for the upland vs lowland (MWW test: df = 1, p < 0.001) and the midland vs lowland (p < 0.001) but not for the upland vs midland (p = 0.641). Species evenness (Figure 4(c)) decreased with increasing altitude. In general, the lowland area was found to have higher species evenness (Figure 4(d)) indicating less variation in number of species groups, though the variation was not significant (K-W test: df = 40, p = 0.615). Further statistical tests indicated that there was no difference between species evenness between the upland vs midland (MWW test: df = 1, p = 0.297), but observe differences between the upland vs lowland (p = 0.016), and the midland vs lowland (p = 0.002).
3.2. Soil Properties and Tree Species Distribution
Results from DCA indicated an overlap in tree species distribution in part of the upland and midland that were influenced by SOC and MC (Figure 5). Furthermore, ExMg influenced distribution of tree species mainly in the midland with some overlaps in the upland. Exchangeable bases (Ca, Na and K), available P, soil pH and BD indicated influence on distribution of lowland species.
![]()
![]()
Table 3. On-farm tree species abundance and their uses on slopes of Mount Kilimanjaro, Tanzania.
Veg. = Vegetation type: SM = Sub-montane vegetation, L = Lowland vegetation as described by (NAFORMA 2010), Abun = Abundance, Freq = Frequency, Distr. index = Distribution index, Abundant > 20, Occasional = 10 - 20, Rare = 0 - 10 (Abundance = total appearance of species in the entire study plots, Frequency = the number of plots to which the species is present, Distribution index = Abundance + Frequency).
![]()
Figure 3. Species distribution (a) showing species spread with sites (species list in Table 1). Species accumulation curves (b) indicating species turnover in the study site on slopes of Mount Kilimanjaro, Tanzania.
![]()
Figure 4. The relationship between altitude and land use with (a) - (b) species richness; and (c) - (d) species evenness. (a) and (c) are regression plots fitted with lines of best fit; (b) and (d) are box and whisker’s plots representing the median values per plot.
![]()
Figure 5. A triplot from a DCA of trees on farm on the slopes of Mount Kilimanjaro, Tanzania. Soil properties are represented by arrows. Polygons are the land uses categories. Three letters are the abbreviations of tree species (full list Table 1). Small square boxes are plots within the land use systems.
4. Discussion
4.1. Tree Species Diversity
Composition of tree species in the study area indicated a mixture of indigenous and exotic species almost in equal proportions (Table 3). While most of the indigenous species were remnants of natural forests, the exotic species were mainly from recent introductions. Botanical studies in the farmland in Kilimanjaro have identified most species (e.g. Table 3) to be forest species [38] , while key exotic species like Avocado (Persea americana) were introduced in ca. 100 years ago [22] [39] . Tree were found to have multiple uses and their retention and/planting on farm has been common in other mountainous areas of Tanzania. Farmland further serves for ex-situ conservation of important tree species [8] . This study identified one species (Table 3) Prunus africana which has been listed as Vulnerable by the IUCN [40] , due to its overexploitation in the wild for traditional medicine through debarking. Furthermore, trees on farm in the study site forms an important corridor connecting various forest patches including large blocks of Kilimanjaro Forest Reserve in the upper part and Kahe I & II Forest Reserves in the lowland, which are about 30 km apart. Habitat connectivity is very important in ensuring unrestricted movement of the genetic material across major habitats [41] .
The study observed distinct tree species communities associated with the three main land use systems in the region (Figure 3(a)). Heavy overcrowding of tree species was found in the upland and part of midland. This indicated that these tree species were closely related to one another and were influenced by similar conditions. The midland area indicated steepest rate of species accumulation (Figure 3(b)) indicating that high turnover was experienced in this zone than others. However, the causes of decline of species richness in upland are not clearly known (Figure 4(a) & Figure 4(b)). Probably, one factor might be less activities of tree seeds dispersal agents in the upland. Higher presence of fruit-eating bats was noted [42] in the midland areas of the study site compared to the lowland and upland. This might have attracted more dispersal across various land uses but with main effect in the midland. The activity of dispersal agents has been crucial in establishment and effective colonization of tree species in landscapes [43] . In the contrary, the lowland contained few species but with higher number of individuals per species (Figure 4(c) & Figure 4(d)). This might have indicated dominance of certain tree species due to adaptation to local factors, especially the saline conditions in the lowland.
4.2. Soil Properties and Tree Species Distribution
Soil properties (Figure 5, Table 2) were found to explain the distribution of the tree species in the study site among the major land use systems. The DCA indicated higher spread in the lowland and higher clustering in the upland and the midland areas. Lowland plant communities showed high positive loadings along Axis-1, whilst upland plant communities had shown negative loadings, with little overlap between the two. While some soil properties exerted positive correlations (SOC & MC), the rest indicated negative correlation. This implies that affinity and tolerance to soil properties differ among tree species. For instance, distribution of Acacia xanthophloea was mainly found in the lowland where water-logged condition was predominant due to frequent floods in compacted soils that do not allow easy infiltration of water.
Strong and positive relationship for tree species with SOC and MC (Table 2, Figure 5), may explain higher concentration of the of tree species in the upland and midland. The increase in precipitation boost both soil moisture content and soil biota activities, which in turn enhance soil organic carbon. Improved SOC and MC attracts considerable number and varieties of tree species due to conducive environment for establishment and growth. Studies have shown that sensitivity of tree species to change in precipitation resulting into more growth in wet areas [44] . Similarly, soil properties especially moisture remained one of influential factor in other mountainous areas in Tanzania in defining vegetation zones (e.g., [45] ). Furthermore, some tree species when established in high SOC soils have shown to contribute its stocks, as noted for Acacia mearnsii, contrary to other species in similar conditions [46] .
A combination of soil properties such as soil pH and exchangeable bases (ExCa, ExK and ExNa) was noted to have negative correlation with tree species (Table 2, Figure 5) in the lowland. Generally, the soil condition in the lowland was very saline due to higher concentration of the exchangeable bases, which risen the soil pH. This affected nutrient uptake by tree species and hence influenced their establishment. Similar observation was made in Brazilian savannah where tree species distribution and nutrient uptake was influenced by soil pH [47] . Saline conditions are known to hinder root activities of tree species, hence limit their establishment and growth [48] . De-salinization in order to reduce salty conditions in the lowland may probably be part of the solution to improve suitability of tree species to establish and grow.
Soil BD was found to have negative correlation with tree species in the lowland (Table 2, Figure 5).The BD increased with decreasing altitude, where lowland had higher BD compared to the upland and midland. Lowland soil BD was 0.7 ± 0.03 (g∙cm−3), which is within range that may cause growth-limiting conditions [49] . The restrictions of BD may include impairing root activities of the tree species and hence limit their capability of uptake of nutrients and water to support growth. However, despite higher BD, yet plant roots may grow due to biopores which offers alternative means to plants to access water and nutrients [50] . Therefore, only those plant tolerant to high BD and/or capable of using alternative means such as biopores may excel in colonizing the lowland areas of the study site.
5. Conclusion
Tree species in the study site were very diverse and provided multipurpose use to the local population. The distribution of the tree species was influenced by soil properties. Three distinct tree species groups were observed aligned and overlapped with land use zones. Soil properties varied with land use zones due to inherently parent material, bioclimatic processes and land management. This variation was responsible for soil conditions which were diverse and thus differed in accommodating tree species. SOC and MC correlated positively to large number of tree species especially in the upland and midland. In the lowland, soil pH, available P, BD and exchangeable bases (ExCa, ExK and ExNa) correlated negatively to tree species and became restrictive to others. Only ExMg was key soil nutrient in the midland and correlated positively with tree species. A likelihood existed that change in soil properties including addressing salinity, SOC and MC in the lowland could improve favourable conditions to accommodate spread of more tree species. This generated information has wide application in conservation strategies of trees on farm.
Acknowledgements
This work was part of PhD program funded by the project titled “The Climate Change Impacts on Ecosystem Services and Food Security in Eastern Africa (CHIESA)”. CHIESA was funded by the Ministry for Foreign Affairs of Finland, and coordinated by the International Centre of Insect Physiology and Ecology (ICIPE) in Nairobi, Kenya. World Agroforestry Centre and CGIAR’s CRP program on Humid Tropics supported Mathew Mpanda in various capacities. The authors are also grateful to the editor and reviewers for their suggestions towards completion of this paper.
NOTES

*Corresponding author.