Ultrasonic Activation of Suzuki and Hiyama Cross-Coupling Reactions Catalyzed by Palladium ()
Received 12 January 2016; accepted 11 April 2016; published 14 April 2016

1. Introduction
Due to the urgent need for solutions to the increasing environmental problem of pollution, awareness has recently grown to limit all its sources as much as possible. Hence, chemists have sought to change the conventional methods of organic synthesis so as to make them more efficient and environmentally friendly. This lies within the framework of a new vision of chemistry known as “green chemistry”. To do so, new synthesis strategies and activation methods are established to adhere to regulations around the world. Among the eco-compatible activation methods we can mention temperature, light, pressure, ultrasound and microwave as physical activation methods, phase transfer catalysis as a chemical activation method, and enzymatic catalysis as a biochemical activation method. These new unconventional methodologies involve green chemistry, which is a developing discipline involving selective high-yield catalysis, solvent-free syntheses that are economical in terms of energy and raw material, clean processes and biodegradable materials.
This research work is interested in studying bi-activation through ultrasonic bias [1] - [10] coupled with the phase transfer catalysis [11] - [16] of some organic reactions. Thus, we propose to investigate the effects of ultrasonic wave cavitation (mono activation) combined with the effects of phase transfer catalysis on the performance of Suzuki [17] - [24] and Hiyama [25] - [35] reactions.
2. Materials and Methods
All compounds were characterized by IR, 1H NMR spectra, 13C NMR spectra and mass spectra. The IR spectra were recorded in KBr with a JASCO FT-IR-420 spectrometer, with a precision of ±2 cm−1 in the 400 - 4000 cm−1 range. The 1H NMR spectra (400 MHz) and 13C NMR spectra (100 MHz) were obtained on a Bruker AC300 spectrometer using CDCl3 as solvent and TMS as an internal standard. Chemical shift is given in ppm.
The coupling products were analyzed by GC-MS (Hewlett-Packard computerized system consisting of a 5890 gas chromatograph coupled with a 5971A mass spectrometer) using fused-silica-capillary columns with a polar stationary phase: Supelcowax 10 (60 m × 0.2 mm × 0.20 Ø film thickness). GC-MS analyses were obtained using the following conditions: carrier gas He; flow rate 1 ml/min; split 1:20; injection volume 0.1 ll; injection temperature 250˚C; oven temperature programmed from 60 to 220˚C at 4˚C/min and holding at 220˚C for 30 min; ionization mode used was electronic impact at 70 eV.
PL spectra were measured on a C6PbI4 thin film using a double monochromator U1000 equipped with a photomultiplier. The excitation wavelength was the 325 nm (3.815 eV) line of a Spectra-Physics beamlock 2085 Argon laser.
Melting points were taken on a Reichert-Heizbank apparatus.
3. Results and Discussion
In the present research work, the study of some Suzuki and Hiyama coupling reactions using arylboronic acids (2a), trimethoxy(Aryl)silane (4a-4b) and aryl bromides (1a) [36] [37] is described. The catalytic system used is chloride Bis (acetonitrile) dichloropalladium (II)/triphenylphosphine (PdCl2(MeCN)2/PPh3) in a basic environment and in the presence of a solvent. The coupling product is formed either under ultrasonic irradiation or by magnetic stirring.
3.1. Effects of Solvents on the Suzuki Reaction between 1a and 2a
As was found in [5] , at a constant temperature and pressure, any reaction has a well-defined activation energy. Thus, in order to change this reaction, a specific activation must be introduced into the reaction system. Such specific activation is intended to decrease ΔG# or provide enough energy for the system to access the transition state surmounting the potential barrier. Within the framework of this hypothesis, the 1a and 2a were allowed to react in the presence of a palladium catalyst in various solvents. The influence of the polarity of the solvent on the yield of this reaction is illustrated in Table 1.
As can be seen in Table 1, the solvents used are those most commonly found in the literature. Indeed, Suzuki coupling requires slight or moderate polar solvents, the nature of which is decisive for the yield. However, the yields tend to decrease when moving from a polar to a nonpolar solvent. A higher dielectric constant is associated with better performance. The separating power of the solvent is directly related to the dielectric constant and has the effect of accelerating the coupling process. Thus, through the dissociation of basic cation-anion binding, the use of a solvent with a high dielectric constant leads to significant activation of the basic unit. In the alcohol solvents the yields are above 40%. The Suzuki reaction, however, seems to be slightly slower in the case of toluene (8%) than in that of methanol (47%). In the presence of a water/DMF mixture, a very good selectivity is obtained in both cases (58% and 52% in v/v = 1:1 v/v = 1:2, respectively). The mixture of two water-formal- dehyde solvents is generally considered as the best solvent for this reaction. These solvents are likely to interact with the 2a by complexation, i.e. the oxygenated compounds have a high affinity for boron derivatives, thus facilitating the transmetalation step.
3.2. Effect of Basic Catalysts on the Suzuki Cross-Coupling Reaction
The aim of studying the impact of the base nature on the evolution of the Suzuki reaction between 2a and 1a in
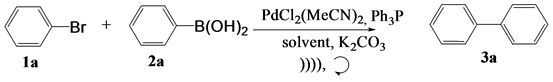
![]()
Table 1. The results of the Suzuki coupling reaction between 1a and 2a in various solvents.
aReaction conditions: PdCl2(MeCN)2 (0.02 mmol), 1a (1.0 mmol), 2a (1.5 mmol), K2CO3 (2 mmol), PPh3 (0.6 mmol) and solvent (3 mL) at 100˚C for 8h; bPdCl2(MeCN)2 (0.02 mmol), 1a (1.0 mmol), 2a (1.5 mmol), K2CO3 (2 mmol), PPh3 (0.6 mmol) and solvent (3 mL), Ultrasonic irradiation for 5 min; *Determined by means of GC, based on the 1a, yields in parenthesis are those of purified products.
water/DMF is to provide some additional elements to the process. That’s why several trials involving various types of basic catalysts were made.
The analysis of the results in Table 2 reveals that the Suzuki coupling reaction catalyzed by palladium using 1a by magnetic stirring at 100˚C did not occur in the absence of a base. Therefore, the yield was found to depend on the nature of the base used. This is in line with previous findings [5] . Besides, the various bases studied are of the type: Et3N, NaOH, KOH, Na2CO3, K2CO3, Cs2CO3, K3PO4 (Table 2). The reactivity increased with the addition of inorganic bases such as KOH, Na2CO3, K2CO3 and Cs2CO3. It was observed that these carbonate anions were more effective and gave yields exceeding 40%. Nevertheless, the addition of organic bases induces slightly lower yields than those obtained in the presence of inorganic ones. In contrast, the obtained results have shown that the bigger the size of the alkali metal cation associated with the carbonate anion is, the more the biphenyl reactivity increases. In fact, the cation size has a direct impact on the value of the reticular energy of the carbonates considered.
As a result, in the presence of these bases, the free carbonate anion in the environment is insufficiently activated, which leads to the weakening of the base cation-anion attraction force [5] . Thus, the larger the increase of the alkali cation size the more significant the carbonate ion reactivity. The size of the alkali cation also leads to the formation of a more nucleophilic quaternary boronate species allowing for transmetalation. Carbonate-type bases are known to be effective during Suzuki coupling, which, when performed under nitrogen pressure, do not
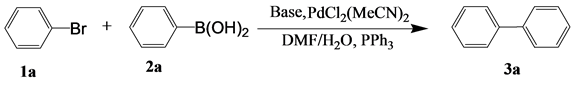
![]()
Table 2. Influence of the nature of the base on the evolution of the Suzuki reaction involving 1a and 2a.
aReaction conditions: PdCl2(MeCN)2 (0.02 mmol), 1a (1.0 mmol), 2a (1.5 mmol), base (2 mmol), PPh3 (0.6 mmol), water, (1.5 mL), DMF (1.5 mL), 100˚C, 8 h; *Determined by means of GC, based on the 1a, yields in parenthesis are those of purified products.
alter their efficiency. Indeed, the use of cesium or potassium carbonate provides good conversions with a slight advantage to Cs2CO3. The opposite-ion, however, does not seem to affect the selectivity for it was found to be perfect and identical in both cases.
3.3. Effect of Ultrasound on the Suzuki Coupling Reaction
The present research work undertakes the study of the effect brought by the ultrasounds on the yield of Suzuki reactions involving 1a-1h and 2a-2p in a water /dimethylformamide mixture and using Cs2CO3 as a base.
The results reported in Table 3 show that the obtained yield is relatively small (of the order of 35%) in the absence of sonochemical activation although the reaction time is relatively long (8 h). Nonetheless, the reactivity increases reaching a yield of the order of 84% in a very remarkable time of 5 minutes in the presence of ultrasonic waves. Indeed, thanks to the physical effects of the cavitation, resulting in the spray of Cs2CO3 powder, a better contact between the solid-liquid heterogeneous environments is created in the presence of ultrasound in the reaction environment. Furthermore, this phenomenon provides considerable energy to the reaction, and hence the synthesis of 3a-3p with good yields (77% - 93%) can be obtained. It is noticed that not only are the yields significantly improved, but also the insulated products under ultrasound have an increased purity. The acceleration of the reaction under ultrasound is probably due to the chemical effects of cavitation which consists in bubbles forming in the liquid and which undergo an implosion after their growth.
These bubbles constitute chemical microreactors in which very high temperature and pressure values in the final stage of their implosion are reached causing the high release of energy. This allows for the stirring of the reaction environment, an activation of the catalytic system and the nearing of reactants by increasing their specific surface area and mixing the liquid layers located near them. This leads to an increase in the contact between the reactants promoting the formation of the product for a very short period of time and with very high yields.
3.4. The Cross-Coupling Hiyama Reaction between 1a and 4a in the Presence of a Base and a Soluble Catalyst Such as PdCl2(MeCN)2/Ph3P
3.4.1. Effects Linked to the Solvent
In order to examine the effect of the solvent type on the course of the Hiyama reaction [38] , a reaction of 1a with 4a [35] [39] was performed in the presence of a catalyst in various solvents. The effect of solvent polarity on the yield of this reaction is illustrated in Table 4.
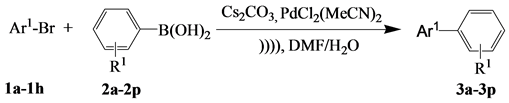
![]()
![]()
Table 3. The yields obtained in the presence of ultrasound or in its absence.
aReaction conditions: PdCl2(MeCN)2 (0.02 mmol), 1a-1h (1.0 mmol), 2a-2p (1.5 mmol), Cs2CO3 (2 mmol), PPh3 (0,6 mmol) , water (1.5 mL) in DMF (1.5 mL) at 100˚C for 8 h; bPdCl2(MeCN)2 (0.02 mmol), 1a-1h (1.0mmol), 2a-2p (1.5mmol), Cs2CO3 (2mmol), PPh3 (0.6 mmol), water (1.5 mL) in DMF (1.5 mL), Ultrasonic irradiation 5 min, 25˚C; *Determined by means of GC, based on the 1a-1h; yields in parenthesis are those of purified products.
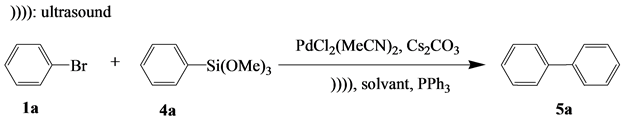
![]()
Table 4. Effect of solvent on the reactivity of the Hiyama reaction under ultrasounds irradiation and magnetic stirring.
THF: tetrahydrofurane, EtOH: ethanol, DMSO: dimethylesulfoxyde, DMF: N, N-dimethylformamide; aReaction conditions: PdCl2(MeCN)2 (0.02 mmol), 1a (1.0 mmol), 2a (1.5 mmol), base (2 mmol), PPh3 (0.6 mmol), water (1.5 mL) in DMF (1.5 mL), 100˚C, 8 h bultrasounds for 5 min; *Deter- mined by means of GC, based on the 1a, yields in parenthesis are those of purified products.
Table 4 shows the Hiyama coupling reaction involving 1a and 4a in different solvents at different temperatures.
The impact of the organic co-solvent on the organic solvent system/H2O (1:1) and the relationship with the solvent mixture DMF/H2O was studied (Table 4). Among the aqueous binary systems, it is worth mentioning that dimethylformamide (DMF) is the most effective co-solvent. Aprotic polar solvents such as acetonitrile (CH3CN), dimethylesulfoxyde (DMSO) and tetrahydrofuran (THF) lead to lower yields (entries 10, 11 and 13). In the presence of an aprotic polar solvent, the coupling yield is not improved (entry 9). A binary system is required for the catalysis as confirmed by the increase in the yield (entries 11, 12). The DMF/H2O (1:1) mixture (entry 12) is considered the best system in the Hiyama coupling. It is worth noting that the reaction in the aqueous environment does not allow for the best coupling (entry 3); and that when toluene was used no product was detected (entry 7).
In the light of these results, the assessment of the 5a coupling was studied using the DMF/H2O solvent system (1:1).
3.4.2. Ultrasonic Activation of Hiyama Reactions in the Presence of Aliquat-336
To illustrate the effect of ultrasound and phase transfer catalysts on the Suzuki reaction trials (Table 5) were carried out involving the coupling of the 1a-1d, 1f, 1h, 1i-1k with 4a, 4b in a mixture of water and dimethylformamide. The first trial was performed under magnetic stirring, the second one under ultrasonic irradiation and the last one under ultrasonic irradiation in the presence of a phase-transfer catalyst.
When working under standard conditions the yield was found to be relatively low. However, in the presence of ultrasound and/or with Aliquat-336 the reaction was almost complete. The acceleration of the reaction under ultrasound is probably due to the chemical effects of cavitation which consists in the formation of bubbles in the liquid and which undergo an implosion after their growth. These bubbles constitute chemical microreactors in which very high temperatures and pressures are reached in the final stage of their implosion are reached causing a high release of energy. This allows for the stirring of the reaction environment, the activation of the catalytic system and the nearing of reagents by increasing their specific surface area and mixing the liquid layers located near them. The increase in the contact between the reactants can therefore be induced, which promotes the formation of the product in a very short time and with very high yields. The set of phenomena, stirring, pressure, temperature, ionization, etc., generated by ultrasounds leads to the disruption of the classical reaction mechanisms. Hence, very high yields have been observed under ultrasounds in the water-DMF environments and in the presence of phase transfer catalysts.
However, the anionic and sonochemical bi-activation also produces purer products with higher yields, which significantly increase if ultrasound is used with a remarkable decrease in reaction time (8 hours to 5 minutes). This can be explained by the fact that ultrasounds improve the contact of heterogeneous liquid-solid environments through the effects of microemulsion also providing energy to the reaction. Nonetheless, there is not much difference between the yields obtained when using ultrasound and those using both the ultrasound and Aliquat-336. Indeed, given that the reaction is almost complete in the presence of ultrasounds, the phase-transfer catalyst no longer has a big role to play.
The yield of these reactions significantly improved, reaching 80%, in the simultaneous presence of ultrasound and Aliquat-336 in a record time of 5 minutes. Indeed, as shown in Scheme 1 that displays the reaction mechanism, the ion exchange reaction between Cs2CO3 and Aliquat-336 induces the formation of an ammonium hydroxide ion which becomes lipophilic, and thus soluble in the organic phase. Furthermore, the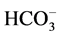 , +N(Oct)3Me ion couple is less associated than the Cs+,
, +N(Oct)3Me ion couple is less associated than the Cs+, 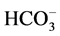 ions, which leads to an exaltation of the basicity of the
ions, which leads to an exaltation of the basicity of the 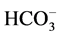 ion in the case where the used ion pair is
ion in the case where the used ion pair is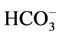 , +N(Oct)3Me. The reactivity order of the hydroxide ions was seen to increase with the increase in the cation size.
, +N(Oct)3Me. The reactivity order of the hydroxide ions was seen to increase with the increase in the cation size.
To further evidence these results, we propose the most likely reaction mechanism for this Hiyama coupling reaction in the two-phase water/dimethylformamide environment in the presence of TCP (Scheme 1).
3.5. PL Properties of Pd Aliquat-336
Scheme 2 illustrates the photoluminescence spectrum of Pd Aliquat-336. The PL spectra of the prepared samples are obtained as a result of the competition among electron-hole separations, electron-phonon scattering and electron-hole recombination. Two emissions are observed in the PL spectra, the first one is a weak blue emission located at about 420 nm and is attributed to the carbon chain, which is due to trap the state emission based on the large Stokes shift from the band gap energy. The second emission at 470 nm may be due to the recombination between the shallow donor level (S vacancy) and the t2 level of the Pd2+ ion.
4. Experimental
4.1. Classical Procedure without Ultrasound
The 1a (1.0 mmol), 2a (1.5 mmol), Cs2CO3 (2 mmol) and the catalyst PdCl2(MeCN)2) (0.02 mmol), PPh3 (0.6 mmol) were placed in a Schlenk tube. Vacuum was applied for 30 minutes, and then argon was admitted. Water (1.5 mL) and N, N-dimethylformamide (1.5 mL) were added. The reaction was carried out at 100˚C for 8 h. After reaction, the mixture was cooled and the organic phase was extracted (three times) with diethyl ether. The
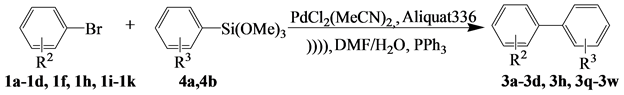
![]()
Table 5. Synthesis of biaryl via Hiyama reaction of trimethoxy (Aryl) silane and aryl bromides under ultrasonic irradiation.
Aliquat-336: N-Methyl-N, N, N-trioctylammonium chloride; cReaction conditions: PdCl2(MeCN)2 (0.02 mmol), 1a-1d, 1f, 1h, 1i-1k (1.0 mmol), 4a, 4b (1.5 mmol), Aliquat-336 (1.25 mmol), Cs2CO3 (2mmol), PPh3 (0.6 mmol), DMF(1,5 mL) and water (1.5 mL), Ultrasonic irradiation at 25˚C for 5 min. *determined after purification by chromatography.
latter was dried on MgSO4 and the solvent removed under vacuum. The coupling product was finally isolated by silica gel chromatography.
Ultrasonic Irradiation
The ultrasonic probe was immersed directly in the reactor. An ultrasonic generator (sonics VC 505 300 W)
![]()
Scheme 1. Proposed mechanism for the Hiyama coupling in the water/DMF biphasic system in the presence of Aliquat-336.
![]()
Scheme 2. PL emission spectrum of Pd Aliquat-336 prepared in a mixture of water and DMF.
emits the sound vibration into the reaction mixture. Sonification was achieved at low frequencies of 20 kHz (amplitude of 50%) at room temperature for 5 min. The 1a (1.0 mmol), Aliquat-336 (1.25 mmol), 2a (1.5 mmol), Cs2CO3, PPh3 (0.6 mmol) and the catalyst PdCl2(MeCN)2) (0.02 mmol) are placed in a reactor. Water (1.5 mL) and N,N-dimethylformamide (1.5 mL) are added. After reaction, the mixture is extracted (three times) with diethyl ether. The latter is dried on MgSO4 and the solvent removed under vaccum. The coupling product is finally isolated by silica gel chromatography.
The yields of the reactions were determined by gas chromatography on a Shimadzu 2014-GC apparatus. The capillary column was DB-5 and the carrier gas was helium.
4.2. Hiyama Couplings
General Procedure for the Syntheses of Biaryl
PdCl2(MeCN)2 (0.02 mmol) was added to a solution of 1a (1.0 mmol), 4a-4b (1.5 mmol), Aliquat-336 (1.25 mmol), PPh3 (0.6 mmol) in a mixture of dimethylformamide (1.5 mL) and water (1.5 mL). The reaction mixture was heated to reflux for 8 h. After the reaction, the mixture was cooled and the organic phase was extracted (three times) with water/hexane (1:1). The latter was dried on MgSO4 and the solvent removed under vacuum. The coupling product was finally isolated by silica gel chromatography.
Biphenyl (3a, C12H10)
White solid; m.p.: 66˚C - 68˚C; 1H NMR (CDCl3): δ = 7.63 - 7.62 (m, 4H), 7.50 - 7.45 (m, 4H), 7.4 - 7.35 (m, 2H) ppm; 13C NMR (CDCl3): δ = 127.23, 127.31, 128.81, 141.32 ppm; IR (KBr): ῡ = 3032, 1946, 1568, 1475, 1427, 902, 729, 694 cm−1; MS: m/z (%) = 154 (M+, 100), 153 (42), 149 (74), 85 (40), 71 (50).
4-Methylbiphenyl (3b, C13H12)
m.p.: 47˚C - 48˚C; 1H NMR (CDCl3): δ = 7.51 (d, 2H, J = 8 Hz), 7.44 (t, 2H, J = 7 Hz), 7.34 (t, 1H, J = 7 Hz), 7.26 (d, 2H, J = 8 Hz), 7.57 (d, 2H, J = 7 Hz) 2.44 (s, 3H, CH3) ppm; 13C NMR (CDCl3): δ = 21.1, 126.8; 127.1, 128.6, 129.4, 137.1, 138.3, 141.1 ppm.
4-Methoxybiphenyl (3c; C13H12O)
Colorless solid; m.p.: 85˚C - 86˚C; 1H NMR (CDCl3): δ = 3.85 (s, 3H), 6.97 (d, 2H, J = 9 Hz), 7.28 - 7.35 (m, 1H), 7.41 - 7.46 (m, 2H), 7.52 - 7.58 (m, 4H) ppm; 13C NMR (CDCl3): δ = 159.4, 141.5, 134.1, 129.0, 128.3, 127.0, 126.7, 114.4, 55.6 ppm; MS: m/z (%) = 184 (M+, 92.5), 169 (77.5), 150 (45.0), 141 (70), 139 (40), 131 (30), 115 (55), 99 (32.5), 91 (52.5), 76 (100), 63 (55.0), 50 (42.5).
4-Nitrobiphenyl (3d, C12H9NO2)
Pale yellow powder; m.p.: 115˚C - 116˚C; 1H NMR (CDCl3): δ = 7.45 - 7.53 (m, 3H), 7.63 - 7.66 (m, 2H), 7.77 (d, 2H, J = 6.9 Hz), 8.33 (d, 2H, J = 6.9 Hz) ppm; 13C NMR (CDCl3): δ = 145.8, 139.4, 132.7, 129.3, 128.7, 128.0, 127.5, 119.1; 111.1 ppm; MS: m/z (%) = 199 (M+, 35.6), 169 (28.9), 153 (19.3), 152 (100), 127 (20.7), 102 (13.3), 76 (49.6), 50 (40).
4-Biphenylcarbaldehyde (3e, C13H10O)
mp.: 58˚C - 60˚C; 1H NMR (CDCl3): δ = 10.05 (s, 1H), 7.98 - 7.96 (m, 2H), 7.78 - 7.75 (m, 2H), 7.68 - 7.64 (m, 2H), 7.52 - 7.49 (m, 2H), 7.47 - 7.44 (tt, 1H, J = 7.2, J = 1.2 Hz) ppm; 13C NMR (CDCl3): δ = 192.1, 147.4, 140.0, 135.5, 130.5, 129.2, 128.7, 127.9, 127.6 ppm; IR (KBr): ῡ = 3059, 3032, 2924, 2828, 2735, 1700, 1604, 1565, 1515, 1485, 1450, 1412, 1384, 1308, 1281, 1214, 1170, 1076, 1007, 917, 838, 762, 729, 697, 646, 629, 547 cm−1; MS: m/z (%) = 183 (M+, 18), 182 (M+, 59), 181 (65), 180 (53), 153 (51), 152 (100), 150 (26), 77 (13), 76 (36).
4-Acetylbiphenyl (3f, C14H12O)
Colorless crystals; m.p.: 117˚C - 119˚C; 1H NMR (CDCl3): δ = 8.03 (d, 2H, J = 8.4 Hz), 7.74 (d, 2H J = 8.4 Hz), 7.62 - 7.64 (m, 2H), 7.34 - 7.49 (m, 3H), 2.64 (s, 3H) ppm; 13C NMR (CDCl3): δ = 197.8, 146.0, 140.4, 136.4, 129.2, 129.1, 128.5, 127.3, 127.3, 26.8 ppm; MS: m/z (%) = 196 (M+), 181, 152, 76, 43.
4-Acetoxybiphenyl (3g, C14H12O2)
Pale yellow solid; m.p.: 114˚C - 116˚C; 1H NMR (CDCl3): δ = 8.07 - 8.03 (dt, J = 8.4, J = 2 Hz, 2H), 7.72 - 7.69 (dt, J = 8.4, J = 1.6 Hz, 2H), 7.67 - 7.63 (m, 2H), 7.51 - 7.48 (m, 2H), 7.45 - 7.41 (tt, J = 7.6, J = 1.2 Hz, 1H), 2.67 (s, 3H) ppm; IR (KBr): ῡ = 2925, 2855, 1679, 1602, 1484, 1361, 1265, 1181, 1083, 960, 838, 765, 722, 680 cm−1; MS: m/z (%) = 196 (M+, 8), 184 (10), 167 (36), 149 (100), 83 (82), 77 (20).
4-Acetyl-4'-methoxybiphenyl (3h, C15H14O)
White crystals; m.p.: 153˚C - 154˚C; 1H NMR (CDCl3): δ = 7.64 (d, J = 8.4 Hz, 2H), 7.54 (d, J = 8.7 Hz, 2H), 7.02 (d, J = 8.7 Hz, 2H), 8.01 (d, J = 8.4 Hz, 2H), 3.89 (s, 3H), 2.62 (s, 3H) ppm; MS: m/z (%) = 226 (M+, 44), 211 (100), 183 (30.5), 168 (28), 152 (33.5), 139 (65.5), 89 (21.6), 77 (20), 63 (40.9), 55 (27.2).
4-Chloro-4'-methoxybiphenyl (3i, C13H11ClO)
White crystals; m.p.: 112˚C - 114˚C; 1H NMR (CDCl3): δ = 7.47 - 7.51 (m, 4H), 7.37 (d, J = 8.4 Hz, 2H), 6.97 (d, J = 8.7 Hz, 2H), 3.86 (s, 3H) ppm; 13C NMR (CDCl3): δ = 159.3, 139.2, 132.5, 128.8, 128, 127.9, 125.9, 114.3, 55.3 ppm; MS: m/z (%) = 220 (M++2, 37.7), 218 (M+, 88.7), 203 (66), 175 (62.3), 152 (47.2), 139 (56.6), 111 (24.5), 101 (30.2), 87 (49.1), 75 (67.9), 63 (79.2), 57 (100).
4-Methoxy-4'-nitrobiphenyl (3j, C7H11NO3)
Yellow powder; m.p.: 105˚C - 106˚C; 1H NMR (CDCl3): δ = 8.27 (d, J = 8.7 Hz, 2H), 7.70 (d, J = 8.7 Hz, 2H), 7.58 (d, J = 8.7 Hz, 2H), 7.04 (d, J = 8.7 Hz, 2H), 3.89 (s, 3H) ppm; 13C NMR (CDCl3): δ = 160.4, 147.2, 131.1, 128.5, 127.0, 124.1, 114.6, 55.4 ppm; MS: m/z (%) = 229 (M+, 100), 199 (25.4), 183 (13.9), 168 (23), 139 (49.8), 63 (23.2).
4-Acetylbiphenyl (3k, C14H12O).
1H NMR (CDCl3): δ = 8.01 - 8.05 (m, 2H), 7.70 - 7.60 (m, 4H,), 7.50 - 7.38 (m, 3H), 2.63 (s, 3H); 13C NMR (CDCl3): δ = 197.9, 146.0, 140.1, 136.1, 129.2, 129.1, 128.5, 127.5, 127.4, 26.8; MS: 196 (M+), 181, 152, 76, 43.
4,4′-Dimethoxybiphenyl (3l, C14H14O2)
Colorless crystals; m.p.: 175˚C - 176˚C; 1H NMR (CDCl3): δ = 7.51 (d, J = 8.7 Hz, 4H), 6.98 (d, J = 8.7 Hz, 4H), 3.88 (s, 6H) ppm; 13C NMR (CDCl3): δ = 158.8 133.8, 127.6, 114.5, 55.7 ppm; MS: m/z (%) = 214 (M+, 93.4), 199 (100), 171 (36.8), 156 (25), 128 (48.7), 115 (17.1), 102 (28.9), 91 (39.5), 74 (32.9), 63 (47.4), 51 (38.2).
4,4’-Dichlorobiphenyl (3m, C12H8Cl2)
white solid; m.p.: 150˚C; 1H NMR (CDCl3): δ = 7.46 (d, J = 8 Hz, 4H), 7.39 (d, J = 7.6 Hz, 4H) ppm; 13C NMR (CDCl3): δ = 127.2, 128.0, 132.7, 137.4 ppm.
4, 4’-Dimethylbiphenyl (3n, C14H14)
White solid; m.p.: 124˚C - 125˚C; 1H NMR (CDCl3): δ = 7.51 (d, J = 8.1 Hz, 4H), 7.26 (d, J = 8.1 Hz, 4H), 2.42 (s, 6H) ppm; 13C NMR (CDCl3): δ = 136.8, 129.7, 138.5, 127.0, 21.3 ppm.
4,4’-Dicyanobiphenyl (3o, C14H8N2 )
white solid; m.p.: 234˚C; 1H NMR (CDCl3): δ = 7.75 - 7.82 (m, 4 H), 7.68 - 7.74 (m, 4 H) ppm; 13C NMR (CDCl3): δ = 112.4, 118.4, 127.9, 132.8, 143.5 ppm.
4-Chlorobiphenyl (3p, C12H9Cl) Viscous liquid; m.p.:77˚C - 78˚C: 1H NMR (CDCl3): δ = 7.55 (m, 4H), 7.41 (m, 5H) ppm; 13C NMR (CDCl3): δ = 140.0 135.6, 133.3, 128.9, 128.8, 128.4, 126.9, 127.6 ppm; IR (KBr): ῡ = 1420, 1114, 1061, 892, 803 cm−1.
5. Conclusion
The present research work has undertaken the study of the bi-activation of some coupling reactions by phase transfer catalysis (PTC) coupled with ultrasounds. The effect of phase transfer catalysis associated with ultrasound waves on the reactivity of certain organic reactions such as Suzuki and Hiyama was therefore also examined. The obtained results have demonstrated that Suzuki reactions are significantly favored in the presence of ultrasound in an aqueous environment. The use of Aliquat-336 plays an important role in the reduction of Pd(II) as well as in the stabilisation and solubilisation of Pd(0).
Acknowledgements
We greatly acknowledge financial support of the Ministry of Higher Education and Scientific Research of Tunisia.
NOTES
![]()
*Corresponding author.