Antioxidant Activity of the Natural Flavonoid 7-Hydroxy-5,6,4’-trimethoxyflavone Isolated from the Leaves of Lippia rugosa A. Chev ()
Received 29 September 2015; accepted 19 February 2016; published 22 February 2016

1. Introduction
Free radicals are atoms or molecules that possess unpaired electrons. They are naturally produced under aerobic conditions but an excess of free radicals can damage all cellular macromolecules including proteins, carbohydrates, lipids and nucleic acids [1] . Studies have also indicated that free radicals are implicated in the pathogenesis of diabetes, liver damage, atherosclerosis, inflammation, cardiovascular disorders, neurological disorders and in the process of aging acids [1] [2] . Antioxidants are substances that neutralize the harmful free radicals in human bodies. They act as free radical scavengers and hence prevent or slow damage done by these free radicals. Their function is as a reducing agent, which ultimately removes free radical intermediates and prevents further oxidation by being oxidized themselves. It has been shown that antioxidants are present in all plants and all parts of the plant. Fruits and vegetables are also known as good sources of antioxidants such as retinol (vitamins A), ascorbic acid (vitamin C), α-tocopherol (vitamin E), glutathione, carotenoids, flavonoids, tannins and other phenolic compounds. Many studies show that daily consumption of fruits and vegetables is associated with reduced risk for degenerative diseases like cancer and cardiovascular diseases. Some antioxidants can be synthesized within the body, but most in human bodies are obtained through diet or supplements. In recent years, interest has increased in finding natural occurring antioxidants for foods or medicinal material to replace synthetic antioxidants which are being restricted due to their carcinogenicity. Much attention has been devoted to natural antioxidant and their association with health benefits. Plants phenolic are commonly found in both edible and non edible plant and have been reported to have multiple biological effects including antioxidant activity. The importance of natural phenolic compounds from plant materials is also raising amount scientists, food manufacturers and consumers due to the specific health effect because of its numerous biological activities: antimicrobial, anticancer; antioxidants, insecticidal etc..., therefore plants derived antioxidant are now receiving special attention. Lippia rugosa A. Chev. (Verbenaceae) is a robust woody perennial plant of the Verbenaceae family up to 12 feet high with large oblong-lanceolate bluish-green leaves; the pleasant aromatic fragrant flowers are white or lilac, small, and whitish in branched inflorescence. Found from Guinea to West Cameroon, L. rugosa growed typically on waste ground and is largely distributed in the savannah area of the Adamawa region of Cameroon [3] . Its habitus is similar to Lippia mulitflora Moldenke; it is locally named “Gossolhi” in Fulfulde and used for medicinal purposes against indigestion, rheumatism, fever, cough and jaundice. Ethnobotanic studies and preliminary surveys revealed that Lippia rugosa is traditionally used on the Vogel Peak Massif for anchoring the roof of round-huts, as roasted fish ingredient in the west region and also for the preservation of stocked foodstuffs and grains in the granaries in the Sahelian regions of Cameroun [4] [5] . For this last purpose fresh leaves are introduced into grain bans to preserve stored cowpea and maize from insects and fungi damages [5] [6] . It is a potential source of essential oil in Cameroon and other tropical areas [5] - [7] . Essential oils from L. rugosa tested for insecticidal activity indicated its potential fumigant property against Sitophilus zeamais Motsch. (Coleoptera: Curculionidae) [8] . In vitro studies also showed that preparations of Lippia rugosa might be potentially used as antifungal agent against Aspergillus species, which are known as dangerous food poisons due to aflatoxin production [9] [10] . To the best of our knowledge, no phytochemical investigation has been carried on the L. rugosa from Cameroon. In our effort to screen antioxidative extracts of native plants from Cameroon [11] , we evaluated in the present work the antioxidant activity of the leaves extracts of L. rugosa using free radical system and its active organic components which, to the best of our knowledge, have not yet been performed.
2. Material and Methods
2.1. Chemicals
Gallic acid (GA) (Sigma-Aldrich, St Quentin Fallavier, France) was used as reference polyphenolic were purchased from Aldrich, Germany. 2,2-diphenyl-1-picrylhydrazyl (DPPH), butylated hydroxyanisole (BHA) were used as free radical donor and antioxidant, Folin-Ciocalteu reagent and Na2CO3, methanol (PA) from Sigma Germany. All other organic solvents used for the tests were upgrade. Water used was distilled.
2.2. Vegetal Materials
The leaves sample of Lippia rugosa were collected in the month of August 2010 between 7 and 7:30 pm local time in the Guinean type savannah in the neighbourhood of the Ngaoundere University’s campus geographically referenced 13˚22.5E; 07˚25.11N and altitude 1036 m [8] . These data were recorded with a GPS Garmin Geko 301. The plant samples were identified and authenticated by Pr Mapongmetsem JM, botanist and agroforester in the Department of Biological Sciences, University of Ngaoundere. These plants material were cut in smaller pieces, air-dried, pulverized in a traditional mortal and sieved into an average size of 2 mm using kitchen mill.
2.3. Extraction of Plant Material and Isolation of Organic Compounds
Five hundred and fifty grams of air-dried powdered of the leaves of Lippia rugosa were extracted exhaustedly at room temperature (25˚C) with 2200 mL of n-hexane by maceration during 4 hours under mechanical agitation at a speed of 3000 rpm using a Prolabo-228 apparatus. The organic layer was then evaporated to dryness at 40˚C under reduced pressure on a 210-Büchii rotary evaporator. This operation was repeated three times to give 13.16 g of hexane extract representing 1.19% of dry material (weight/weight). The residue was then successively extracted in the same manner with organic solvents of increasing polarity respectively with ethyl acetate; ethanol and methanol to give respectively 4.01 g (0.72%), 7.83 g (1.42%) and 6.2 g (1.12%) of ethyl acetate; ethanol and methanol extracts. Theses extracts were kept at 4˚C until used.
2.4. Preliminary Phytochemical Investigation
Extracts of Lippia rugosa were subjected to preliminary phytochemical screening according to standard procedures. These extracts were tested for the presence of alkaloids, steroids/triterpenoids, phenols, flavonoids, saponins, tannins and carbohydrates [12] [13] .
2.5. Antioxidant Tests Using DPPH Assay
2.5.1. Qualitative DPPH Assay Using Tin Layer Chromatography (TLC)
The qualitative test to DPPH consisted of using a chromatographic TLC plate on which spots of these extracts were spotted and develop in n-hexane-ethyl acetate or ethyl acetate-methanol system. Chromatographic plates were then spread with 0.2% DPPH solution and kept in the dark for 30 min. Spots initially purple became yellow, thus indicating the antioxidant character of different compounds present in some of these extracts [14] . The DPPH test provides information on the reactivity of the test compounds with a stable free radical. DPPH gives a strong absorption band at 517 nm in visible region. When the odd electron becomes paired off in the presence of a free radical scavenger, the absorption reduces and the DPPH solution is decolourised as the colour changes from deep violet to light yellow. The degree of reduction in absorbance measurement is indicative of the radical scavenging power of the extract. The fast colour change is an indication of the high antioxidant potential of the extract or compound.
2.5.2. Quantitative DPPH Assay
The antioxidant capacity was measured according to the method used by Zhang with some slide modifications [15] . A stock solution of 100 μg/mL of each extracts or pure organic compounds were prepared by diluting to 100 μg/mL for a standard range of concentrations: 100, 80, 60, 40, 20 μg/mL. The Butylated hydroxyanisole (BHA) was used as positive control. For each concentration, assays were triplicated, the optical densities or absorbance were measured using a Rayleigh VIS-723N spectrophotometer at 517 nm and antioxidant activities were evaluated using the following formula:
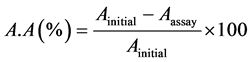 (1)
(1)
where 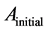 is the absorbance of the control reaction and
is the absorbance of the control reaction and 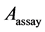 is the absorbance of the test extract or purified organic compound.
is the absorbance of the test extract or purified organic compound.
2.6. Polyphenol Contents
Total phenolic compounds in the leaves of Lippia rugosa were determined with Folin-Ciocalteu using method described by Singleton and Rossi (1965) amended by Dewanto and calculated using gallic acid as the standard compound [16] . The organic extracts were diluted in methanol. Five point concentrations were prepared with Gallic acid as the positive control. Extracts (100 μl) were added to 50% Folin-Ciocalteu reagent (100 μl). After 3 min, 2 ml of 2% Na2CO3 solution was added to the mixture, which was then left to stand for 30 min. Absorbance was measured at 760 nm on a spectrophotometer and compared to gallic acid calibration curves. All analyses were run in three replicates and averaged.
2.7. Isolation of Organic Compound
Twenty grams of the ethanol extract of L. rugosa were fractionated by column chromatography (CC) on silica gel 60S (300 g, 230 - 400 mesh) using mixture of n-hexane-ethyl acetate and ethyl acetate-methanol of increasing polarity as eluent. Thin layer chromatography (TLC) was carried out on silica gel precoated plates F-254 Merck and the spots on the TLC plates were visualized by UV254, UV365 and spraying with 50% H2SO4 following by heating at 105˚C. Fractions of 150 ml each were collected and compared by TLC (silica gel) using n-hexane-ethyl acetate and ethyl acetate-methanol of increasing polarity as eluent and the fractions giving similar spots in TLC were combined. In the end seventeen fractions A-Q were obtained. The fractions J (167 - 173), eluted with pure ethyl acetate, were combined on the basis of thin layer chromatography (TLC) analysis, and the presence of a green precipitate was observed, which after recrystallization with n-hexane/ethyl acetate (10/90) (v/v) gives a purified green organic compound identified as compound LRF4 (60 mg) representing respectively 0.41% and 0.0053% of the ethanol extract mass and dry plant powder (w/w) respectively.
2.8. Spectroscopic Characterization
Chemical characterization of purified active compound was done through 1H and 13C-NMR spectral analyses. NMR spectra were recorded on a Bruker Avance 500 MHz spectrometer. Chemical shift values (δ) are reported in parts per million (ppm) relative to appropriate internal solvent standard and coupling constants (J values) are given in Hertz. Mass spectra were recorded on a Finnigan MAT 95 high resolution, double focusing magnetic sector mass spectrometer. Accurate mass measurement was achieved using voltage scanning of the accelerating voltage. This was nominally 5 kV and an internal reference of heptacosa was used. Resolution was set between 5000 and 10,000. IR spectra were recorded on a Nicolet 360 FT-IR spectrophotometer and UV spectra on a Thermo Electron Corporation Helios spectrophotometer.
2.9. Statistical Analysis
The experimental results were expressed as the mean ± Standard Deviation (SD) using Microsoft Excel 2007. Curves and graphs were obtained by using the software Sigmaplot (version 9.0).
3. Results and Discussion
3.1. Phytochemical Results
The results of the qualitative phytochemical analysis of the leaves extracts of L. rugosa are giving in Table 1.
![]()
Table 1. Results of the phytochemical screening of L. rugosa leaves extracts.
−: Absent; +: Present; ++: Abundant.
They indicated that each plant extract contained at least one class of secondary metabolites, the ethanol and the methanol extracts being the richest extracts containing the greatest number of organic compounds which include flavonoids, polyphenols, sterols and triterpenoids whereas hexane and ethyl acetate extracts contained respectively one and two classes of secondary metabolites. Compounds belonging to terpenoids as well as phenolics are well documented for their biological activities. The presence of the flavonoids and polyphenols compounds in the ethanol and methanol extracts corroborate with previous research works carried out on Lippia alba [17] [18] . Their presence in the studied extracts could therefore explain the observed activities and justified the traditional uses.
3.2. Antioxidant Test
TLC assay of these extracts spraying with 0.2% DPPH in 96% ethanol showed immediately many bleached bands for the ethanol and methanol extracts while no and few bleached bands were observed after hours respectively on the ethyl acetate and n-hexane extracts indicating their poor and no antioxidative activity of these two last extracts. The best activity seen in the ethanol and methanol extracts prompted us to quantify the antioxidant activity of these extracts. The lower and weakest antioxidant activity of the ethyl acetate and the hexane extracts confirmed the absence of phenolic compounds in both extracts.
The results of the antioxidant activities of the L. rugosa extracts and pure organic compound LRF4, measured by DPPH˚ assay are presented on Figure 1 and Figure 2. It was observed from this curve that inhibition values of L. rugosa ethanol and methanol extracts, compound LRF4 and standard BHA increased with concentration. The ethanol extract of L. rugosa is an antioxidant with a percentage of inhibition reaching 85.668% ± 1.233% at the highest concentration (100 µg/mL) than the methanol extract and ethyl acetate. The ethanol extract of L. rugosa was more effective than the pure isolated compound LRF4 isolated from this extract indicating that the total antioxidant of the extract is due to the synergetic effect of many compounds of the extract including those of compound LRF4. High R2 values obtained also showed that there is a good correlation between concentration and radical inhibition. We also note that each concentration, there is a significant difference between the extracted probability threshold p < 5%.
![]()
Figure 1. Inhibition percentages of Butylated hydroxyanisole (BHA) and L. rugosa extracts in (µg/mL).
![]()
Figure 2. Inhibition percentage of compound LRF4 using DPPH assay in (µg/ml).
3.3. Amount of Total Phenolic Compounds
The key role of phenolic compounds as scavengers of free radicals is emphasized in several reports. Amounts of phenolic components of the ethanol extract of Lippia rugosa were found to be 0.0229 ± 0.0003 Gallic acid equivalents (GAEs), similar to those of methanol extract content (Table 2). This result is in agreement with results observed in the antioxidative test showing best result for ethanol and methanol extracts. Although qualitative screening indicated presence of phenolic compounds in ethyl acetate extract, it content in phenolics is very low evaluated at 0.00000 ± 0.00019 GAEs. The phenolic compounds of the ethanol extract may contribute directly to antioxidative action. The high antioxidant activity of the ethanol and methanol extracts could be attributed to their high polyphenolic contents known to be highly rich in antioxidant compounds [16] [19] .
3.4. Identification of Isolated Compound LRF4
Compound LRF4 was obtained as a green amorphous solid which gives positive reaction to phenolic and flavonoid test reactions. Its ESI-TOF mass spectrum showed a molecular ion peak [M+1]+ at m/z 329.5 consistent with the molecular formula C18H16O6 and eleven insaturations. Its UV spectrum showed two absorption bands at λmax in EtOH at 289 and 332 nm corresponding respectively to the benzoyl and cinnamoyl chromophores of a flavonoid compound. The structure of this compound was totally characterized using 1H and 13C NMR spectrophotometric techniques (Table 3). From the analyses, compound LRF4 was identified as 7-hydroxy-5,6,4'-trime- thoxyflavone or 3-(40-methoxybenzyl)-7,8-methylenedioxy-chroman-4-one (Figure 3). The structure of the isolated flavonoid meet partially the second structural requirement stated by Bors et al.(1990) for a flavonoid molecule to have a maximal radical scavenging activity by bearing a 2,3-double bond in conjunction with a 4- oxo group in the C-ring [20] . Spectroscopic characteristics of 7-hydroxy-5,6,4'-trimethoxyflavone (LRF4) is given below:
ESI-TOF MS m/z: 329.5 [M + 1]+; 314.4; 296.5; 268.4; 133.4.
UV λmax (EtOH) nm: 289; 332
1H and 13C NMR see Table 3.
The presence of flavonoids compounds are in agreement with previous results obtained on the genus Lippia. Flavonoid molecules were previously isolated and their antioxidative activity evaluated from L. graveolens [21] . Despite the fact that compound 7-hydroxy-5,6,4'-trimethoxyflavone was previously isolated from the whole plant
![]()
Table 2. Results of the polyphenols contents and antioxidant activities of L. rugosa extracts.
−: not measured; BHA: Butylated hydroxyanisole, ARP: antiradical power.
![]()
Table 3. 1H (500 MHZ, CDCl3) and 13C NMR spectral data of compound LRF4.
of Eupatorium catarium [22] , this is the first time that it was isolated from the genus Lippia and particularly from L. rugosa from Cameroon and its antioxidative activity evaluated. In recent years, it has been demonstrated that phenolic compounds play a widespread role in the natural plant protection against phytophagous insects and phytopathogenic fungi [23] . The presence of phenolic compounds and 7-hydroxy-5,6,4'-trimethoxyflavone could also explain the traditional uses of L. rugosa to protect maize against pests like Sitophillus zeamais Motsch and Prostephanus truncatus and f against ungi like Fusarium [8] [9] .
4. Conclusion
The aim of this work was to evaluate the antioxidant activity of the various extracts of Lippia rugosa (Verbenaceae) growing in Ngaoundere (Adamawa region, Cameroon) and its secondary’s metabolites. Results obtained
![]()
Figure 3. Structure of compound LRF4 identified as 7-hydroxy-5,6,4'-trimethoxyflavone.
showed that its ethanol extract was the most antioxidant with the flavonoid 7-hydroxy-5,6,4'-trimethoxyflavone with a low antioxidative activity being, one of the secondary metabolites responsible of this activity. These results, which to the best of our knowledge constitute the first report on the antioxidative activity of the L. rugosa organic extracts, provide some support for its traditional uses in the treatment of degenerative diseases, as a potential source of antioxidant compounds and open a window for an in-depth anti-infectious study of the plant and other related species of the genus Lippia.
Acknowledgements
J. MOMENI thanks the International Foundation of Science (IFS) for the material from grant F/4895.
NOTES
![]()
*Corresponding author.