Estimating Potential Nitrogen Mineralisation Using the Solvita Soil Respiration System ()
Received 23 November 2015; accepted 18 December 2015; published 23 December 2015

1. Introduction
Nitrogen (N) and phosphorus inputs are said to be the primary nonpoint sources of pollution in many parts of the United States [1] . Plants obtain N from fertilizer applications, atmospheric deposition, precipitation, and biological fixation and mineralisation. Fertilizer recommendations are routinely based on the amount  determined during soil testing, negating the contribution of N due to biological activity and resulting in over application of fertilizer. Long-term use of fertilizer has a negative environmental impact by contributing to excessive N and P loading in streams, rivers, and ultimately the ocean. It is therefore important to be able to accurately predict N mineralisation in the environment; however, accurate soil N mineralisation estimates are difficult to obtain rapidly in the laboratory setting [2] .
determined during soil testing, negating the contribution of N due to biological activity and resulting in over application of fertilizer. Long-term use of fertilizer has a negative environmental impact by contributing to excessive N and P loading in streams, rivers, and ultimately the ocean. It is therefore important to be able to accurately predict N mineralisation in the environment; however, accurate soil N mineralisation estimates are difficult to obtain rapidly in the laboratory setting [2] .
The mineralisation of soil N is controlled by microbial activity as they decompose organic material to obtain energy. Microbial activity is dependent upon soil temperature and moisture, agricultural practices, and physical and chemical soil characteristics. Smith and Humfeld [3] observed that during decomposition studies of green manures, bacteria counts trended with CO2 evolution, rising rapidly during the first 4 days and then declining to a constant level. Previously in 1919, Gainey [4] had noticed the parallel formation of CO2, NH4-N, and NO3-N in soil. Lebedjantzev [5] indicated that drying soil at a low temperature in the laboratory or the field appeared to increase the fertility of the soil. CO2 respiration from soil has been studied for over 90 years as an indicator of the rates of C, N, or P mineralisation and ultimately the relative fertility of various soils [4] - [6] . It is now commonly accepted N mineralisation is a measurement of soil quality [7] that is dependent upon the biogeochemical cycling systems of soils including microbial activity.
The Solvita Soil Respiration System (Solvita) is a new tool to evaluate soil microbial respiration rates in an efficient and cost-effective manner, without the need for reagent handling and long incubation periods. Solvita was designed as a complete procedure to quantify the relative differences between varying types of compost by measuring the amount of CO2 evolved in a short time period [8] . The amount of CO2 evolved is interpreted as an indication of the completeness of active degradation, also called a maturity index. In composts and manures, Solvita has been used in combination with other indices to determine compost maturity and C and N mineralisation (see www.Solvita.com for complete publications list). The Solvita system has also been suggested as a potential tool for assessing N mineralisation in soils and is currently being utilized by several commercial and government laboratories throughout the United States for this purpose [9] -[11] .
There are many published methods for the determination of N mineralisation including long-term aerobic incubation (3 days and 24 days [12] , 41 weeks [13] ), 7-day anaerobic incubation [14] , in situ field methods [2] , and others [15] . These methods are either too time consuming and laborious for commercial labs to perform at a cost effective price or are not adequate for predicting N mineralisation.
The 7-day anaerobic N mineralisation method is considered the best biological indicator of potentially available N and is commonly used to compare new N-mineralisation testing methods [15] . The objective of this study is to apply the Solvita measurement of CO2 in soils to determine N mineralisation and compare it to the 7-day anaerobic N mineralisation method developed by Waring and Bremner [14] .
2. Materials and Methods
A random sub-sampling (207) of soil samples from the contiguous United States and England, UK that were submitted to the United States Department of Agriculture-Agricultural Research Service lab in Temple, TX, from 2010 to 2014 were utilized in this study. Samples were obtained from Texas, Idaho, Maine, West Virginia, Oklahoma, Georgia, and Louisiana. Some samples obtained were not specifically identified when submitted to the laboratory. In addition, soil-testing results from 50 samples provided by an independent private soil-testing lab were utilized, bringing the total number of samples in the study to 257. The locations for the samples provided from the soil-testing lab were not provided. Methods utilized by both labs were identical.
Soil samples were obtained from the top 15 cm of the soil profile. All soil samples were dried at 50˚C for 24 h and ground to pass a 2-mm sieve. Soil organic matter content was determined using loss on ignition [16] . Ten- gram soil samples were analyzed prior to incubation and extracted with 40 ml of 2 M KCl to determine initial  content. Nitrogen mineralisation was determined using the anaerobic incubation test described by Waring and Bremner [14] , and equals the
content. Nitrogen mineralisation was determined using the anaerobic incubation test described by Waring and Bremner [14] , and equals the  detected on an OI Analytical RFA after incubation minus the
detected on an OI Analytical RFA after incubation minus the 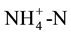 initially present in the soil.
initially present in the soil.
The flush of CO2-C following rewetting of dried soil was determined in 40-g subsamples of each soil in 50-ml polypropylene disposable beakers (Fisherbrand Cat. No. 01 - 291 - 10) with four to five 6.35-mm holes drilled in the bottom. A whatman GF/D 4.25-cm glass microfiber filter (Cat No. 1823 - 042) was placed in the bottom of the beaker to prevent soil loss. The beaker and Solvita Soil Respiration paddle were placed in a gas-tight 250-mL glass jar filled with 20 mL of water and sealed with a screw-top lid. The glass jar has a convex bottom to allow for drainage. Capillary action was used to rewet soil according to its water holding capability [17] . Soils were incubated at 25˚C and respired CO2-C was measured after 24 h. The quantity of CO2-C released was determined using a digital-color reader (DCR) as described by Haney [9] . The Solvita system for estimating the flush of CO2-C following rewetting of dried soil has been shown to be highly correlated with the commonly used titration method and CO2-C IRGA method [9] . SigmaStat imbedded in SigmaPlot ver. 12.2 was used for linear regression analysis [18] .
3. Results and Discussion
The range in soil organic matter for the soil samples was 0.8% to 7.5% (Figure 1). The Solvita results in mg C per kg soil are highly correlated with anaerobic 7-day N mineralisation values (Figure 2). These data correspond with findings by [12] , [19] [20] that the flush of CO2 following rewetting dried soil is strongly correlated with potential N mineralisation. While both the anaerobic 7-day mineralisation test and the Solvita test do not require preliminary analysis to determine the amount of water added to each soil, the 7-day N mineralisation test does require preliminary 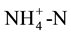 determination and Solvita does not. In addition, the Solvita test can be performed without the use of reagents to extract
determination and Solvita does not. In addition, the Solvita test can be performed without the use of reagents to extract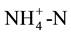 , laboratory steam distillation and titration (which introduces considerable human error), and can be completed in just over 24 h versus 7 days. Furthermore, Wienhold et al. [2] indicate that anaerobic N mineralisation underestimates field N mineralisation when compared to aerobic N mineralisation. The ability to rapidly assess potential N mineralisation with a simple test should be highly attractive to commercial soil testing labs.
, laboratory steam distillation and titration (which introduces considerable human error), and can be completed in just over 24 h versus 7 days. Furthermore, Wienhold et al. [2] indicate that anaerobic N mineralisation underestimates field N mineralisation when compared to aerobic N mineralisation. The ability to rapidly assess potential N mineralisation with a simple test should be highly attractive to commercial soil testing labs.
One possible limitation to predicting N mineralisation from the anaerobic test or directly from Solvita is that, regardless of the respiration number, you cannot predict when a given soil is in a state of N immobilization. Only in situ field mineralisation tests, which are not practical at a large spatial or commercial laboratory temporal scale, would possibly be able to predict field conditions under any given time. The relationship between Solvita and mineralisation will be sufficient to predict field N mineralisation.
4. Conclusions
Based on the strong correlation between the 7-day N mineralisation test and Solvita test results, across a diverse set of soils, it would be justified to utilize Solvita to predict N mineralisation using the equation below (r2 = 0.82):

![]()
Figure 1. Range in soil organic matter (%) in 257 soil samples analyzed.
![]()
Figure 2. Solvita 1-day C vs. 7-day anaerobic N mineralisation from 257 soil samples.
Solvita can be used to rapidly assess soil respiration and relative N mineralisation potential in any given soil under laboratory conditions. The advantage to utilizing the Solvita test is that: the test only takes 1 day versus 7 days to perform and the analytical equipment is less expensive (Solvita machine versus colorimetric analysis). These results are novel in that Solvita is the first test that uses soil-microbe respiration to estimate N mineralization which is, in fact, microbially driven.
NOTES
![]()
*Corresponding author.