Comparative Dosimetric Study for Treating Left Sided Breast Cancer Using Three Different Radiotherapy Techniques:Tangential Wedged Fields, Forward Planned Segmented Filed, and IP-IMRT ()
1. Introduction
The proportion of breast cancer patients treated with radiation therapy (RT) has increased substantially during the past two decades [1] . Multiple, prospective, randomized trials have established the equivalence of breast- conserving therapy in which radiotherapy is an integral component (considered the standard of care for early stage) to mastectomy, regarding locoregional control, disease free and overall survival [2] .
20 year study National Surgical Adjuvant Breast and Bowel Project-06 “NSABP B-06” showed no long term significant difference regarding DFS, OS and distant metastasis free survival between mastectomy and lumpectomy with radiation. Radiation reduces the local failure from 39% to 14% compared to lumpectomy alone [3] .
Early Breast Cancer Trialists’ Collaborative Group meta analysis of 78 randomized trials including 42,000 women showed that RT after BCS, and RT after mastectomy with axillary clearance in LN+ disease, produced significant absolute improvements in 5-year LR (17% - 19% benefit) and 15-year breast cancer mortality (5.4% benefit). RT produced similar proportional reductions in LR risk (~70% risk reduction) irrespective of age, grade, tumor size, ER status, or amount of LN involvement [4] .
So, better local treatment adds to the effect of systemic therapy on LR, which can be translated into a moderate breast cancer mortality benefit. Therefore, the aim of radiation therapy should also be directed to minimize the risk of complications which may develop in critical organs [5] .
In patients with left breast cancer, the critical organs in radiotherapy are: ipsilatreral lung, heart, coronary artery, contralateral lung and contralateral breast. The most often found complications in these patients are cardiac and pulmonary function disorders and development of second malignancies [6] . Hotspots and dose inhomogeneity also lead to poor cosmetic outcomes, especially in women with larger breasts. More skin reactions and desquamation, can lead to pain, fibrosis and reduced breast appearance and decreased quality of life [7] .
Cardiac complications may develop after 10 years following radiotherapy and they are most frequently observed in women with left-sided breast cancer. These complications cause a 30% increase in cardiovascular deaths after the period of 10 years following radiotherapy. Pulmonary complications are confined to antero- lateral peripheral (subpleural) region of the lung on the irradiated side. They are usually divided into early and late complications. Immediately after radiotherapy, patients may develop radiation pneumonitis which later evolves into lung fibrosis [8] - [10] .
Generally, patients with breast cancer are at a higher risk for a second cancer in the contralateral breast. Moreover, radiotherapy of breast cancer is associated with a small, but significant, increased long term risk of contralateral breast cancer (CBC), particularly among women treated under the age of 45 and has strong family history [11] .
Because the risk of radiation-induced second malignancy is a stochastic process with no apparent threshold dose and seems to demonstrate a dose-dependent relationship, this emphasizes the need for reduction of radiation dose to the contralateral breast using asymmetric jaws (MLC) and some form of intensity modulation, avoiding hard wedges, and cerroband half beam blocks [12] .
Several single-institution studies and two randomized trials for breast cancer have reported that field-in-field or forward-planned IMRT technique improves the dose homogeneity and decreases the acute skin toxicity as well as the dose to the contralateral breast and doses to lung and heart compared with conventional tangential techniques with wedges [13] [14] .
This improvement in dose homogeneity has been most remarkable in the treatment of large breasts. Homogeneity also becomes more important in hypofractionation schemes, this simple forward field in field planning method obtained a homogeneous dose distribution, within dose constraints similar to inverse planning IMRT which requires sophisticated technical resources and is more time consuming [15] [16] .
Aim
To compare the dosimetry for the left breast cancer radiotherapy using three different radiotherapy techniques, tangential wedged fields, forward planned segmented filed, and inverse planning IMRT.
2. Material and Methods
Twenty patients with left breast cancer were randomly selected for this treatment planning study. They have undergone breast-conserving surgery and received a prescribed dose of 50 Gy in 25 fractions.
2.1. Target and Normal Tissue Delineation
CT scan was acquired of the patient in the supine position with both arms extended above the head. The target volumes (the whole breast) and sensitive structures, such as the heart, ipsilateral lung, contralateral lung, and contralateral breast, were delineated in 5-mm-thick CT slices.
The breast CTV included all visible breast parenchyma based on wired breast tissue, limited 5 mm from skin and anterior to pectoralis muscle (exclude lung/heart). The PTV was added a 7-mm expansion in all direction around the CTV (set-up margin and patient movement) except the skin surface (no CTV-PTV margin was taken), with exclusion of the heart and anterior to ribs and lung. The CTV of all the 20 cases were delineated based on CT image. The PRV contours of all the involved OARs, including contra-lateral breast, entire heart, both lungs, and coronary artery region.
All plans were completed in three-dimensional treatment planning system (CMS-Xio). Delineation of the heart started at one slice below the pulmonary trunk; and extends inferiorly to the apex of the heart. Both lungs should be contoured using pulmonary windows. The right and left lungs were contoured separately but they should be considered as one structure for lung dosimetry.
The coronary artery most commonly affected by radiation is the left anterior descending, followed by the right branch and left circumflex [17] . The area of left front one-fourth heart 1cm subsurface can be identified as the volume of coronary artery part according to the American Memorial Sloan-Kattering cancer research methods [18] .
2.2. Plan Design
All plans were completed in three-dimensional treatment planning system. The Siemens linear accelerators with 6 MV, 10 MV or 15 MV photon energy were used. The PTV was prescribed to 50 Gy.
The field borders were clinically defined with radiopaque wires during simulation and also delineated according to the location of the tumor, extent of breast tissue, and adequate set-up margins. The field borders extended up to midline medially, lower border of clavicle superiorly, and laterally and inferiorly 2 cm beyond the palpable breast tissue.
To avoid radiation omission in the target region of the lacteal gland caused by respiratory movement, the front limit of the fields was set at 20 mm off the skin surface.
The entrance and exit points of coplanar tangential fields are aligned three dimensionally on the treatment planning work station. The normalization point was defined at approximately mid-depth and 1 cm superficial to the deep edge of the chest wall in the plane of the central axis of the beams.
The TW plan used two opposite half beam which had an appropriate wedge angle and included the whole PTV. The “isocenter” of the treatment machine is positioned at the centre point of the midline joining two parallel opposing fields. Physical or dynamic wedges were then added to both tangential beams in order to improve the dose uniformity to the PTV, and to compensate for the rapid changes in external contours. Dose constraints for tangential setup were a central lung distance (CLD) of <30 mm and a maximum heart distance of <10 mm.
The FIF plan is created with same beam angles of the conventional TW plan. Two parallel opposed tangential fields were designed. Subfields for the medial and lateral beams were designed using the multileaf collimators to ensure the Dmax of PTV less than 55 Gy (<110%) and to shield lung and heart.
Inverse IMRT: 7 fields were designed, with gantry set at 300˚, 330˚, 0˚, 30˚, 60˚, 90˚, and 130˚. After providing some optimizing constraints (shown in Table 1), distribution of dose curves was automatically optimized, and through repeated parameters adjustment, the ideal distribution of dose curves was achieved.
![]()
Table 1. The optimization objective used for inverse IMRT planning.
2.3. Data Analysis
The conformity index (CI) and homogeneity index (HI) were defined to describe the quality of plans as follows:
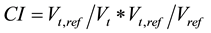
where Vt represents target volume, Vt,ref represents the target volume wrapped by reference isodose curve face (95%), and Vref represents all the volume wrapped by reference isodose curve facev (95%). A higher CI value, ranging from 0 −1, represents better conformity.
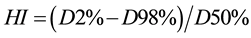
D2%, D50% and D98% mean the doses of 2%, 50% and 98% volume of the PTV, where D2 represents the dose corresponding to 2% target volume as shown in DVH and can be deemed as the maximum dose; D98 represents the dose corresponding to 98% target volume as shown in DVH, and can be deemed as the minimum dose and D50% represent the prescribed dose. Therefore, a lower HI is indicative of a more homogeneous dose distribution across the PTV.
2.4. Evaluation of Planes
The treatment plans generated were compared objectively using the dose volume histograms (DVHs) for PTVs and different Organs at Risk (OARs) regions of interest. In the PTV, the values of V105, V95, V90, Dmax, Dmean, homogeneity index (HI), conformity index (CI) were compared for all these three techniques.
For OAR (heart, coronary artery region and left lung), the dose values of Dmean, V5, V10, V20, V30, were recorded and V3, V2, V1 and V0.5 doses for contralateral breast were evaluated and compared for the three techniques.
2.5. Statistical Analysis
Comparison of the dosimetric characteristics for planning target volume and organs at risk for the 3D-CRT, FiF, and IP-IMRT plans. The results difference between the three plans were compared and analyzed with using SPSS 17.0 software, and p value ≤ 0.05 was considered statistically significant.
3. Results
Figure 1 displays that the mean volume of PTV breast receiving >105% of the prescribed dose was 1.75% for IP-IMRT, 2.03% for FiF, and 4.82% for TW. The difference is statistically significant through paired comparison between FiF vs TW (P ≤ 0.0001) and IP-IMRT vs TW (P ≤ 0.0001) but not statistically significant difference between FiF and IPIMRT (Table 2). Although higher Dmax are observed in TW technique, no statistically significant difference compared with IPIMRT or FiF plannings.
The mean volume of PTV covered by 95% isodose (V 95%) was 92.1% for TW, 96% for FiF, and 95.1% for inverse planning IMRT. The difference is statistically significant through paired comparison between FiF vs TW (P ≤ 0.0001) and IP-IMRT vs TW (P ≤ 0.0001) with no statistically significant difference between FiF and IP-IMRT as shown in Figure 2 (Table 2).
Better conformity and homogeneity indices are noted in Figure 3 and Figure 4 for FiF and IMRT compared to TW the difference is statistically significant (P ≤ 0.0001) through paired comparison, but no statistically significant difference between FiF and IPIMRT (P = 0.0675) and (P = 0.2134) (Table 2).
![]()
Figure 1. Comparison of mean value of PTV receive ≥105% of the prescribed dose for the 3 planes.
![]()
Figure 2. Comparison of mean V95% of PTV for the 3 planes.
![]()
Figure 3. Comparison of mean homogeneity index for the 3 planes.
![]()
Figure 4. Comparison of mean conformity index of the 3 planes.
![]()
Table 2. The PTV dose parameters of three plans.
Regarding organs at risk, for the left lung, higher values of V5, V10, and V20 for IP-IMRT compared to TW and FiF. The differences are statistically significant through paired comparison with no statistically significant difference between FiF and TW (Table 3).
Figure 5 showed that the left lung mean dose (Dmean) was 13.93 Gy for IP-IMRT compared to 7.26 Gy for FiF and 8.73 Gy for TW fields, the differences are statistically significant through paired comparison (P < 0.0001) with no statistically significant difference between FiF and TW (P = 0.141). Regarding V30, no statistically significant difference observed throughout the three techniques paired comparison (Table 3).
Regarding the heart, Figure 6 revealed that V5, V10, V20 and Dmean were significantly higher for IP-IMRT compared to FiF and TW fields (P < 0.0001), While lower mean V30 values for IP-IMRT compared to FiF and TW fields, the differences were statistically significant through paired comparison in favor of IPIMRT (P < 0.0001), with no statistically significant differences between FiF and TW (P = 0.3401) (Table 3).
For the coronary artery region, as shown in Figure 7, higher V5 and V10 values for IP-IMRT compared to FiF and TW but the difference is not statistically significant, lower V20 and V30 for IP-IMRT compared to FiF or TW plans with statistically significant differences throughout paired comparison (P ≤ 0.0001) (P = 0.0015) with no statistically significant difference in the mean dose (Dmean) between FIF and IP-IMRT (P = 0.0950).
Concerning the contra lateral breast, FiF and TW significantly decrease the dose (Dmean, V0.5, V1, V2, and V3) significantly compared to IP IMRT with no statistically significant difference between FiF and TW plans. This is quite evident in Figure 8 (Table 3).
![]()
Table 3. Organs at risk dose parameters.
![]()
Figure 5. Comparison of Dmean of the left lung for the 3 planes.
![]()
Figure 6. Comparison of Dmean of the heart for the 3 planes.
![]()
Figure 7. Comparison of Dmean of the coronary artery region for the 3 planes.
![]()
Figure 8. Comparison of Dmean of the contralateral breast for the 3 planes.
4. Discussion
The objective of this study was to compare the dosimetry for the left breast cancer radiotherapy using three different radiotherapy techniques, tangential wedged fields, forward planned segmented fields, and inverse planning IMRT.
Intensity-modulated radiation therapy (IMRT) has been described to improve the target conformity and dose homogeneity in treating breast cancer. Advantages in treating the intact breast have been noted for IMRT with respect to dose homogeneity especially for large breasts and doses to the lung and heart.
It was shown that the FiF-results were comparable to the IP IMRT technique in terms of breast coverage, delivering 95% of the prescribed dose to >95% of the breast PTV, Dmax, the mean dose delivered to the breast PTV, the same CI and HI with no statistically significant difference in paired comparison. IP-IMRT and FIF compared with the TW resulted in a significantly smaller hot spot within the breast volume (V105%), significantly reduced the maximum dose and significantly better conformity and homogeneity indices.
The results of our study match with other similar studies. Efficacy of FIF technique versus tangential field is clearly brought out by Sasaoka and Futami [19] . The field-in-field technique significantly reduced the maximum dose, the volumes receiving more than 107% of the prescription dose, and improves the HI for the PTV for dose evaluation compared with the tangential field technique. Inverse planning-IMRT plan had obvious advantages on the HI than TW plan, but it was not superior to the FIF plan.
For each dosimetry of the OARs, (heart, coronary artery region and left lung), the field-in-field technique significantly reduced the maximum dose and the volumes receiving more than 5, 10, 20 Gy of the prescribed dose compared to IP-IMRT. The volume of the contralateral breast receiving up to 3 Gy was significantly decreased using the field-in-field or TW techniques compared to inverse planning IMRT.
The application of IP-IMRT offers the potential for improved local-regional control without increase heart toxicity in those requiring local-regional treatments.
Xu et al. conjectured the cardiac dose might be associated with the breast volume for whole left breast irradiation. In their report IMRT treatment could significantly reduce cardiac dose for those clinical target volume (CTV) larger than 500 cc compared with conventional tangential techniques [15] .
Darby S.C. et al. reported that exposure of the heart to ionizing radiation during radiotherapy for breast cancer increases the subsequent rate of ischemic heart disease [20] . Most of the literature analyzed the irradiated dose of heart, but they did not specify the dosimetric parameters of coronary artery when comparing the dose difference of treatment plans for the left sided breast cancer.
Radiation tolerance of the coronary arteries has not been well studied beyond the observation that radiotherapy (RT) doses used for treatment of Hodgkin lymphoma increase the risk of coronary artery disease (CAD). Historically there have been recommendations to use the dose volume histogram (DVH) for the heart as a whole organ. This assumed that the heart was an organ with “parallel subunit” toxicity, in that tolerance was acceptable if only a small volume of heart received a high RT dose as long as most of the heart received a low dose. This may be falsely reassuring if we do not know whether the small volume of high dose happens to include the coronary artery [21] .
Coronary arteries are really a “series subunit” organ like the spinal cord. Damage to any portion of a coronary artery will produce potentially devastating toxicity even if the rest of the artery is not irradiated. Therefore heart DVH determinations are of little usefulness to estimate risk of CAD.
This was clearly demonstrated by Taylor et al., patients treated for left breast cancer with tangential RT fields may have a low mean heart dose and a very broad range of doses to the left anterior descending artery (LAD) branches [22] .
The TW and FiF-techniques required fewer monitor units (MU) to deliver a given dose, compared with IP- IMRT. In the IP-IMRT, the number of MUs is found to be about three to four times greater than in the FiF-plans, or TW plans. Increased MU results in excess of machine and treatment times as well as higher machine leakage and total body stray radiation dose, increasing the volume of normal tissues exposed to the very low dose. This could result in a greater probability of radiation-induced complications and secondary malignancy.
The IP-IMRT for breast treatment is time consuming and requires advanced planning skills. In contrast to the 3D-TW and FiF plans, the IP-IMRT required pretreatment verification and specific quality assurance (QA) measurements. The additional QA time must be taken into account when considering the total workload per plan. Compared with the IP-IMRT, FiF-plans and TW plans are likely to be generated in a shorter time without requiring a high level of planning ability.
5. Conclusion
Field in Field technique for tangential whole breast radiotherapy is an efficient and reliable method for achieving a uniform dose throughout the whole breast. Strict dose-volume constraints can be readily achieved in most patients, resulting in improved breast coverage, potential sparing of risk organs and reduction of acute and late toxicities.
Conflict
There is no conflict of interest.
Approved
The study is approved by the ethics committee Faculty of Medicine, Alexandria University, Egypt.
NOTES
*Corresponding author.