Catalyst and Solvent-Free Microwave Assisted Expeditious Synthesis of 3-Indolyl-3-hydroxy Oxindoles and Unsymmetrical 3,3-Di(indolyl)indolin-2-ones ()
1. Introduction
Indoles and their derivative oxindoles are a privileged class of molecules in synthetic as well as biological chemistry. The hybrid molecules of indole and oxindole are also important due to their prevalent biological activities. For instance, the spermicidal activity of 3,3-bis(5-methoxy-1H-indol-3-yl)indolin-2-one and 3,3-bis(3-car- boxymethyl-1H-indol-2-yl)indolin-2-one is higher than that of the standard spermicide “Nonoxynol-9” (N-9) [1] ; di(indolyl)indolin-2-one derivatives showed strong and selective cytotoxicity against cancer cells [2] . These molecules are also known to possess antibacterial, anti-inflammatory and antiprotozoal activities [3] . In addition to these manifold biological applications, very recently 3-indolyl-3-hydroxy oxindoles (hybrid molecule of isatin and indole) were used as precursors in the synthesis of natural products (+)-gliocladin [4] and (+)-folicanthine [5] and in the synthesis of the heterocyclic analogue of BINAP [6] . The synthesis of 3-indolyl-3-hydroxy oxindoles involves a Friedel-Crafts type of electrophilic substitution between the electron-rich third position of the indole and electron deficient carbonyl group of the isatin [7] . This reaction usually results in the formation of symmetrical 3,3'-diindolyl oxindoles in a single step. Controlling the reaction at monosubstituted 3-indolyl-3-hydroxy oxindole stage is quite challenging.
The synthesis of symmetrical 3,3-di(indolyl)indolin-2-ones has been reported [8] - [22] , while in the case of unsymmetrical 3,3-di(indolyl)indolin-2-ones, so far only three reports are available in the literature to the best of our knowledge. The synthesis involves a stepwise process. Wang and Ji reported an ultrasound irradiation method in the presence of ceric ammonium nitrate (CAN) for 1 to 5 h [23] ; Moghadam and co-workers reported the reaction in the ionic-liquid N,N,N,N-tetramethylguanidinium trifluoroacetate (TMGT) for 1 h [24] and Nikpassand and co-workers reported it with a montmorillonite in 30 - 35 minutes [25] .
In the case of 3-indolyl-3-hydroxy oxindoles, Kumar and co-workers reported the synthesis of various 3-in- dolyl-3-hydroxy oxindoles by supramolecular catalysis (β-Cyclodextrin) in 45 - 190 minutes [7] ; Shanthi and co-workers reported a time of 60 - 120 minutes using K2CO3 as a catalyst [26] ; Meshram and co-workers reported a time of 15 minutes in the presence of Triton B [27] ; Hosseini and Tavakolian reported a reaction time of 2.5 h using ZnO nanorods in aqueous medium [28] . EtOH and water medium (60:40) in the presence of Lewis acid (FeCl3∙6H2O) and ultrasonic irradiation in 5 - 25 minutes was reported by Khorshidi and Tabatabaeian [29] while Makarem and co-workers reported an electrochemical method using EtOH/Propanol (60 - 240 minutes) [30] . Srihari and Murthy reported a heterogeneous catalyst (Kaolin/KOH) in the presence of MeOH as a solvent in 138 - 470 minutes [31] . Using various ionic-liquids, Moghadam and co-workers reported a reaction time of 10 - 20 minutes [24] . Jing Deng and coworkers reported an enantioselective version by using cupreine [32] . Nadine and coworkers also reported the reaction using chiral scandium(III) and indium(III) pybox complexes [33] . Recently, Pravathaneni Sai Prathima and co-workers reported the synthesis in aqueous medium using a base catalyst-diethanolamine [34] . The focus of our lab is to develop “green” methods for organic synthesis. In addition to using biocatalysts for organic transformations, we have now used microwave irradiation for organic synthesis as reported in the present study. Use of solvent-free and catalyst-free reaction conditions is an attractive proposition as seen in numerous microwave-assisted reactions. To the best of our knowledge, this is the first report for catalyst and solvent-free synthesis of 3-indolyl-3-hydroxy oxindoles and unsymmetrical 3,3- di(indolyl)indolin-2-ones under microwave irradiation in 5 and 10 minutes respectively.
2. Results and Discussion
Optimization of reaction conditions for the synthesis of 3-indolyl-3-hydroxy oxindoles, was carried out using a isatin (1a) [1 equivalent] and an indole (1b) [1.2 equivalents]. Using a microwave oven temperature at 100˚C and irradiation for 5 minutes, gave only the symmetrical 3,3-di(indolyl)indolin-2-ones as product (Scheme 1). Decreasing the time of irradiation to 3 minutes and then to 1 minute also resulted in the formation of the symmetrical 3,3-di(indolyl)indolin-2-one product and in 1 minute, 3-hydroxy-3-(1H-indol-3-yl)indolin-2-one (1c) and unreacted starting materials isatin (1a) and indole (1b), were also detected. Then we envisioned that 100˚C was not a suitable temperature for controlling the reaction at the mono substituted hydroxyl stage. The reaction was therefore carried out at reduced temperatures: at 90˚C and 80˚C for 5 minutes, a mixture of 3-hydroxy-3-
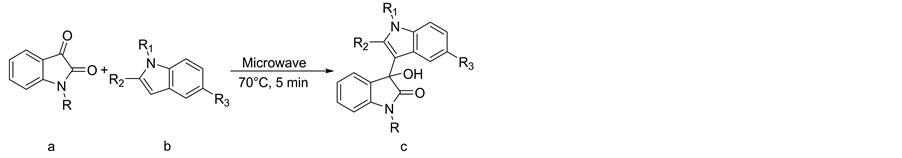
Scheme 1. Synthesis of various 3-indolyl-3-hydroxy oxindoles.
(1H-indol-3-yl)indolin-2-one (1c), symmetrical 3,3-di(indolyl)indolin-2-one in addition to unreacted starting material were observed. At 70˚C for five minutes, only 3-hydroxy-3-(1H-indol-3-yl)indolin-2-one (1c) and unreacted starting materials were detected. But an increase in the reaction time from the sixth minute onwards, resulted in the formation of symmetrical 3,3-di(indolyl)indolin-2-one, even at 70˚C. Based on this observation, 70˚C and 5 minutes were optimized as the reaction conditions for synthesizing 3-hydroxy-3-(1H-indol-3-yl) indolin-2-one (1c), but the isolated yield was only 31%. Further increasing the amount of one of the starting materials i.e. three and five equivalents of indole (1b), showed no improvement in the yield of 3-hydroxy-3- (1H-indol-3-yl)indolin-2-one (1c). Also with N-allyl, and N-benzyl isatins, the yields of N-allyl-3-hydroxy-3- (1H-indol-3-yl)indolin-2-one (5c) and N-benzyl-3-hydroxy-3-(1H-indol-3-yl)indolin-2-one (9c) were only 34% and 40% respectively. Based on the nucleophilicity index of the indole ring [35] , N-methyl indole, 2-methyl indole and 5-methoxy indole were selected [these are electron-rich at the third position]. In addition, 5-nitro indole which is electron deficient at the third position was also selected. The reaction between 5-methoxy indole and simple isatin, gave a yield of 64% for 5-Methoxy-3-hydroxy-3-(1H-indol-3-yl)indolin-2-one (3c). Interestingly excellent yields were obtained for products synthesized from the reactions between N-allyl, N-benzyl, N-pro- pargyl and N-methyl isatins and 5-methoxy indole which is electron rich at the third position. Thus, N-al- lyl-3-hydroxy-3-(5-methoxy-indol-3-yl)indolin-2-one (8c), N-benzyl-3-hydroxy-3-(5-methoxy-indol-3-yl) indolin-2-one (12c), N-propargyl-3-hydroxy-3-(5-methoxy-indol-3-yl)indolin-2-one (14c) and N-methyl-3-hydroxy- 3-(5-methoxy-indol-3-yl)indolin-2-one (15c) gave yields of 97%, 98%, 89% and 96% respectively. 76% yield was obtained in the reaction of 2-methyl indole with simple isatin to give 3-hydroxy-3-(2-methyl-1H-indole- 3-yl)indolin-2-one (2c), while N-allyl isatin and N-benzyl isatin gave N-allyl-3-hydroxy-3-(2-methyl-indol-3- yl)indolin-2-one (7c) and N-benzyl-3-hydroxy-3-(2-methyl-indol-3-yl)indolin-2-one (11c) with 90% and 92% yields respectively. Moderate yields were obtained in the case of N-methyl indole with various isatins. The yields were 56% for N-allyl-3-hydroxy-3-(1-methyl-indol-3-yl)indolin-2-one (6c) 56% for N-benzyl-3-hydroxy- 3-(1-methyl-indol-3-yl)indolin-2-one (10c) and 58% for N-propargyl-3-hydroxy-3-(1-methyl-indol-3-yl) indolin-2-one (13c). No product was obtained in the case of electron withdrawing nitro group present at fifth position of the indole ring with simple isatin even after a prolonged reaction time of 10 minutes and at a temperature of 100˚C (Table 1).
The proposed reaction mechanism for the synthesis of 3-hydroxy-3-(1H-indol-3-yl)indolin-2-one (1c) is shown in Scheme 2.
Entry 8 (Table 1) was selected as a representative example for comparing reaction rates in various solvents such as acetonitrile (ACN), 1,4-dioxane, water (H2O), ethanol (EtOH) and 1,2-dichloroethane (1,2-DCE). Moderate yield was observed only in the case of ACN (53%) in five minutes which improved to 96% in a reaction time of 15 minutes. Comparatively less yields were observed in the case of H2O (46%), EtOH (46%), 1,2-DCE (36%) and 1,4-dioxane (15%).
Synthesis of Unsymmetrical 3,3-Di(indolyl)indolin-2-ones
Formation of symmetrical diindolyl was observed as a major product at 100˚C in the synthesis of 3-hydroxy-3- (1H-indol-3-yl)indolin-2-one (1c). The same conditions were adopted for synthesizing unsymmetrical 3,3-di (indolyl)indolin-2-ones. For optimization, 5-methoxy-3-hydroxy-3-(1H-indol-3-yl)indolin-2-one (1d) [1 equivalent] and indole [1.2 equivalents] were selected. At a microwave oven temperature of 150˚C and irradiation for 5 minutes, 53% product 3-(5-methoxy-1H-indol-3-yl)-3-(1H-indol-3-yl)indolin-2-one (1e) was formed. At a temperature higher than 150˚C and a reaction time of 10 minutes, no significant increase in the yield was observed. When the same conditions were applied to the reaction [i.e. 150˚C for 10 minutes] using various N- substituted isatins, good yields were obtained in all the cases (Table 2, Scheme 3).
All the compounds were characterized by IR, 1H, 13C NMR and HRMS. To the best of our knowledge com-
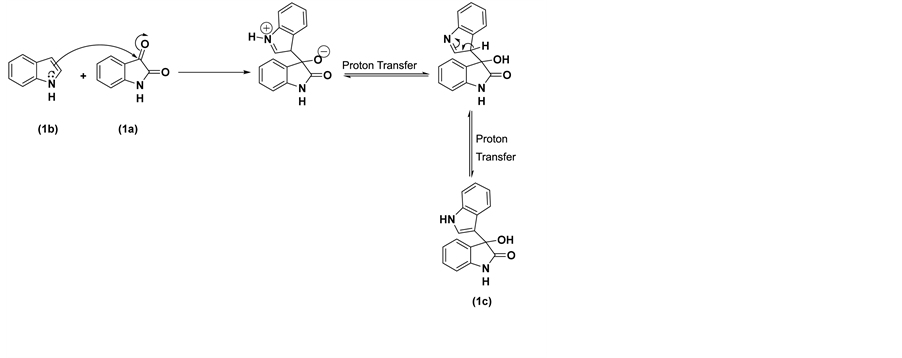
Scheme 2. Proposed mechanism for the formation of 3-indolyl-3-hydroxy oxindole.
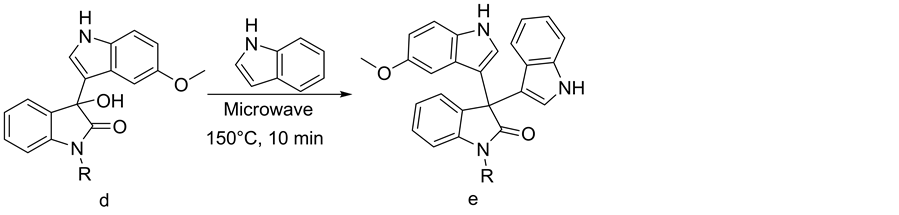
Scheme 3. Synthesis of various unsymmetrical 3,3-di(indolyl)indolin-2-ones.
![]()
Table 1. Synthesis of various 3-indolyl-3-hydroxy oxindoles.
[a] Isolated yield.
![]()
Table 2. Synthesis of various unsymmetrical 3,3-di(indolyl)indolin-2-ones.
[a] Isolated yield.
pounds N-Allyl-3-hydroxy-3-(1-methyl-indol-3-yl)indolin-2-one (6c), N-Allyl-3-hydroxy-3-(5-methoxy-indol- 3-yl)indolin-2-one (8c), N-Benzyl-3-hydroxy-3-(1-methyl-indol-3-yl)indolin-2-one (10c), N-Propargyl-3-hy- droxy-3-(1-methyl-indol-3-yl)indolin-2-one (13c), N-propargyl-3-hydroxy-3-(5-methoxy-indol-3-yl)indolin-2- one (14c); 3-(5-methoxy-1H-indol-3-yl)-3-(1H-indol-3-yl)indolin-2-one(1e), 3-1-methyl(5-methoxy-1H-in-dol-3- yl)-3-(1H-indol-3-yl)indolin-2-one (2e), 3-1-allyl(5-methoxy-1H-indol-3-yl)-3-(1H-indol-3-yl)indolin-2-one (3e), 3-1-benzyl(5-methoxy-1H-indol-3-yl)-3-(1H-indol-3-yl)indolin-2-one (4e) and 3-1-(prop-2-ynyl)(5-methoxy-1H- indol-3-yl)-3-(1H-indol-3-yl)indolin-2-one (5e) are reported here for the first time.
3. Material and Methods
Chemicals were obtained from Spectrochem, and used without further purification. All known products were identified by comparison of their physical and spectral data with those of authentic samples. 1H and 13C NMR spectra were recorded on a Bruker AV-400 spectrometer operating at 400 and 100 MHz, respectively. The spectra were calibrated on the solvent residual peak (DMSO-d6: δ = 2.50 ppm for 1H and δ = 39.52 ppm for 13C; CDCl3: δ = 7.26 ppm for 1H and δ = 77.0 ppm for 13C). Analytical thin layer chromatography (TLC) was performed on Kieselgel 60 F254 aluminum sheets (Merck 1.05554). Column chromatography was performed on silica gel (100 - 200 mesh). FT-IR spectra were recorded on a Nicolet-6700. High-resolution mass spectra were recorded with Thermo Scientific-Orbitrap Elite (Electro spray Ionization). Anton Paar microwave synthesizer was used at 600 rpm and temperature was kept constant at 70˚C and 150˚C.
3.1. General Procedure for the Synthesis of 3-Indolyl-3-hydroxy Oxindoles (1c to 15c)
A mixture of the isatin (0.6797 mmol, 100 mg) and indole (0.8156 mmol, 96 mg) was added to a microwave-oven reaction vial then irradiated for 5 minutes at 70˚C. After completion of the reaction (as indicated by TLC) the residue was washed with 2 mL of DCM and mixed with silica gel and then evaporated the solvent under reduced pressure, and the mixture was purified by column chromatography using EtOAc in hexanes. All the known products have spectral and physical data consistent with those reported in literatures.
3.1.1. 3-Hydroxy-3-(1H-indol-3-yl)indolin-2-one (1c)
White solid; mp: 294 - 296 (lit26: 294˚C - 296˚C). 1H NMR (400 MHz, DMSO-d6): δ 10.98 (s, 1H), 10.34 (s, 1H), 7.35 (t, J = 9.6 Hz, 2 H), 7.27 - 7.24 (m, 2H), 7.09 (d, J = 2.4 Hz, 1H), 7.03 - 7.02 (m, 1H), 6.98 - 6.86 (m, 4H), 6.36 (s, 1H). 13C NMR (100 MHz, DMSO-d6): δ 178.5, 141.7, 136.8, 133.5, 129.1, 124.9, 124.8, 123.5, 121.7, 121.1, 120.3, 118.5, 115.5, 111.5, 109.6, 74.9. IR (KBr): υ 3264, 1711, 1617, 1549, 1467, 1424, 1335, 1226, 1184, 1107, 1071, 938, 910, 745, 686, 655 cm−1. HRMS: Calcd for C16H12O2N2 [M + Na]+ 287.0899, found 287.0795.
3.1.2. 3-Hydroxy-3-(2-methyl-1H-indol-3-yl)indolin-2-one (2c)
White solid; mp: 178˚C - 180˚C (lit26: 176˚C - 178˚C). 1H NMR (400 MHz, DMSO-d6): δ 10.86 (s, 1H), 10.33 (s, 1H), 7.25 - 7.17 (m, 3H), 6.96 - 6.88 (m, 4H), 6.74 - 6.70 (m, 1H), 6.26 (s, 1H), 2.34 (s, 3H). 13C NMR (100 MHz, DMSO-d6): δ 178.7, 141.6, 134.8, 134.1, 133.4, 129.0, 126.6, 124.9, 121.7, 119.8, 119.2, 118.2, 110.2, 109.6, 109.4, 75.8, 13.3. IR (KBr): υ 3348, 3229, 3034, 1693, 1611, 1487, 1454, 1431, 1374, 1345, 1291, 1239, 1211, 1163, 1024, 996, 954, 927, 893,853, 832, 737, 696 cm−1. HRMS: Calcd for C17H14O2N2 [M + Na]+ 301.0948; found 301.0953.
3.1.3. 5-Methoxy-3-hydroxy-3-(1H-Indol-3-yl)indolin-2-one (3c)
White solid; mp: 196˚C - 198˚C (lit26: 196˚C - 198˚C). 1H NMR (400 MHz, DMSO-d6): δ 10.84 (s, 1H), 10.33 (s, 1H), 7.28 - 7.23 (m, 3H), 7.02 (d, J = 2.8 Hz, 1H), 6.98 (t, J = 7.6 Hz, 1H), 6.92 (d, J = 7.26 Hz, 1H), 6.86 (d, J = 2 Hz, 6.71 (dd, J = 2.4 Hz, J = 8.8 Hz, 1 H), 6.34 (s, 1H), 5.76 (s, 1H), 3.62 (s, 3H). 13C NMR (100 MHz, DMSO-d6): δ 178.5, 152.7, 141.7, 133.4, 132.0, 129.0, 125.4, 124.8, 124.2, 121.7, 115.0, 112.0, 110.9, 109.6, 102.7, 75.0, 55.2. IR (KBr): υ 3318, 3166, 1701, 1614, 1581, 1465, 1433, 1344, 1304, 1235, 1207, 1173, 1039, 927, 837, 779, 752, 650 cm−1. HRMS: Calcd for C17H14O3N2 [M + Na]+ 317.0897; found 317.0902.
3.1.4. N-allyl-3-hydroxy-3-(1H-indol-3-yl)indolin-2-one (5c)
White solid; mp: 148˚C - 150˚C. 1H NMR (400 MHz, DMSO-d6): δ 11.01 (s, 1H), 7.35 - 7.33 (m, 4H), 7.06 - 7.04 (m, 4H), 6.89 - 6.86 (m, 1H), 6.49 (s, 1H), 5.89 - 5.82 (m, 1H), 5.24 - 5.16 (m, 2H), 4.38-4.27 (m, 2H). 13C NMR (100 MHz, DMSO-d6): δ 176.4, 142.2, 136.8, 132.7, 132.0, 129.0, 124.9, 124.5, 123.6, 122.3, 121.1, 120.4, 118.5, 117.0, 115.2, 111.5, 109.1, 74.6, 41.4. IR (KBr): υ 3219, 3056, 1688, 1603, 1485, 1459, 1435, 1365, 1332, 1294, 1241, 1223, 1182, 1104, 981, 921, 905, 826, 735, 682 cm−1. HRMS: Calcd for C19H16O2N2 [M + Na]+ 327.1109; found 327.1110.
3.1.5. N-allyl-3-hydroxy-3-(1-methyl-indol-3-yl)indolin-2-one (6c)
White solid; mp: 136˚C - 138˚C. 1H NMR (400 MHz, CDCl3): δ 7.67 (d, J = 8 Hz, 1H), 7.52 (d, J = 7.2 Hz, 1H), 7.33 - 7.19 (m, 3H), 7.09 - 7.05 (m, 2H), 6.97 (s, 1H), 6.90 (d, J = 7.6 Hz, 1H), 5.91 - 5.81 (m, 1H), 5.30 - 5.22 (m, 2H), 4.44 (dd, J = 4.8 Hz, J = 16 Hz, 1H), 4.28 (dd, J = 4.8 Hz, 16.4 Hz, 1H), 3.69 (s, 3H), 3.42 (m, 1H). 13C NMR (100 MHz, CDCl3): δ 176.9, 142.4, 137.7, 131.2, 129.6, 127.7, 125.4, 124.9, 123.1, 122.1, 120.8, 119.7, 117.8, 113.8, 109.5, 109.4, 75.5, 42.5, 32.8. IR (KBr): υ 3349, 3046, 1699, 1606, 1462, 1412, 1365, 1329, 1208, 1178, 1137, 1113, 1072, 984, 934, 888, 743, 669 cm−1. HRMS: Calcd for C20H18O2N2 [M + Na]+ 341.1260; found 341.1261.
3.1.6. N-allyl-3-hydroxy-3-(2-methyl-indol-3-yl)indolin-2-one (7c)
White solid; mp: 164˚C - 166˚C (lit26: 164˚C - 166˚C). 1H NMR (400 MHz, DMSO-d6): δ 10.91 (s, 1H), 7.32 (t, J = 7.6 Hz, 1H), 7.26 (d, J = 7.2 Hz, 1H), 7.19 (d, J = 8 Hz, 1H), 7.01 (t, J = 7.6 Hz, 2H), 6.92 - 6.85 (m, 2H), 6.71 (t, J = 7.2 Hz, 1H), 6.40 (s, 1H), 5.91 - 5.81 (m, 1H), 5.27 - 5.16 (m, 2H), 4.39 - 4.26 (m, 2H), 2.38 (s, 3H). 13C NMR (100 MHz, DMSO-d6): δ 176.6, 142.2, 134.8, 133.7, 133.3, 131.9, 129.0, 126.6, 124.7, 122.4, 119.8, 119.2, 118.2, 117.1, 110.3, 109.1, 75.5, 41.5, 13.3. IR (KBr): υ 3401, 1703, 1607, 1523, 1488, 1461, 1431, 1370, 1331, 1304, 1222, 1181, 982, 931, 895, 759, 673 cm−1. HRMS: Calcd for C20H18O2N2 [M + Na]+ 341.1261; found 341.1266.
3.1.7. N-allyl-3-hydroxy-3-(5-methoxy-indol-3-yl)indolin-2-one (8c)
White solid; mp: 102˚C - 104˚C. 1H NMR (400 MHz, DMSO-d6): δ 10.88 (s, 1H), 7.34 (brs, 2H), 7.24 (d, J = 8.8 Hz, 1H), 7.08 - 7.06 (m, 3H), 6.78 (s, 1H), 6.70 (d, J = 8.4 Hz, 1H), 6.48 (s, 1H), 5.89 - 5.82 (m, 1H), 5.25 (s, 1H), 5.18 (t, J = 10.8 Hz, 1H), 4.32 (dd, J = 14 Hz, J = 35.6 Hz, 2 H), 3.60 (s, 3 H). 13C NMR (100 MHz, DMSO-d6): δ 176.4, 152.8, 142.2, 132.6, 132.0, 131.9, 129.1, 125.3, 124.6, 124.3, 122.4, 117.0, 114.7, 112.1, 111.2, 109.0, 102.2, 74.6, 55.1, 41.5. IR (KBr): υ 3324, 1702, 1611, 1485, 1463, 1435, 1361, 1210, 1177, 1109, 1064, 990, 9224, 899, 840, 805, 752, 704, 674, 631 cm−1. HRMS: Calcd for C20H18O3N2 [M + Na]+ 357.1215; found 357.1202.
3.1.8. N-benzyl-3-hydroxy-3-(1H-indol-3-yl)indolin-2-one (9c)
White solid; mp: 120˚C - 124˚C (lit26: 120˚C - 124˚C). 1H NMR (400 MHz, DMSO-d6): δ 11.03 (s, 1H), 7.37 - 7.24 (m, 9H), 7.10 (s, 1H), 7.05 - 6.96 (m, 3H), 6.82 (t, J = 7.6 Hz, 1H), 6.57 (s, 1H), 4.92 (s, 2H). 13C NMR (100 MHz, DMSO-d6): δ 176.8, 142.1, 136.8, 136.4, 132.7, 129.0, 128.5, 127.4, 124.8, 124.5, 124.5, 123.6, 122.5, 121.1, 120.4, 118.4, 115.1, 111.5, 109.1, 74.7, 42.7. IR (KBr): υ 3296, 3029, 1697, 1607, 1488, 1458, 1428, 1342, 1238, 1212, 1166, 1067, 991, 901, 738, 694 cm−1. HRMS: Calcd for C23H18O2N2 [M + Na]+ 377.1266; found 377.1251.
3.1.9. N-benzyl-3-hydroxy-3-(1-methyl-indol-3-yl)indolin-2-one (10c)
White solid; mp: 126˚C - 128˚C. 1H NMR (400 MHz, DMSO-d6): δ 7.38 - 7.25 (m, 9H), 7.12 - 7.10 (m, 2H), 7.02 (t, J = 7.6 Hz, 1H), 6.97 (d, J = 8 Hz, 1H), 6.87 (t, J = 7.6 Hz, 1H), 6.54 (s, 1H), 4.91 (s, 2H), 3.73 (s, 3H). 13C NMR (100 MHz, DMSO-d6): δ 176.6, 142.1, 137.2, 136.3, 132.6, 129.0, 128.4, 127.9, 127.3, 125.1, 124.5, 122.4, 121.1, 120.6, 118.5, 114.3, 109.6, 109.1, 74.5, 42.7, 32.3. IR (KBr): υ 3302, 1693, 1610, 1549, 1486, 1464, 1373, 1342, 1211, 1164, 1112, 1077, 990, 928, 893, 855, 747, 702, 669, 628 cm−1. HRMS: Calcd for C24H20O2N2 [M + Na]+ 391.1422; found 391.1420.
3.1.10. N-benzyl-3-hydroxy-3-(2-methyl-indol-3-yl)indolin-2-one (11c)
White solid; mp: 96˚C - 98˚C. 1H NMR (400 MHz, DMSO-d6): δ 10.91 (s, 1H), 7.36 - 7.18 (m, 7H), 7.19 (d, J = 8 Hz, 1H), 6.99 (t, J = 7.2 Hz, 2H), 6.92 - 6.88 (m, 1H), 6.80 (d, J = 8 Hz, 1H), 6.68 - 6.64 (m, 1H), 6.47 (s, 1H), 4.92 (ABq, J = 16 Hz, J = 20 Hz, 2H), 2.37 (s, 3H). 13C NMR (100 MHz, DMSO-d6): δ 177.0, 142.1, 136.3, 134.8, 133.7, 133.4, 129.0, 128.5, 127.5, 126.5, 124.8, 122.5, 119.8, 119.2, 118.1, 110.3, 109.1, 109.2, 109.1, 75.6, 42.7, 13.3. IR (KBr): υ 3314, 3288, 3092, 1700, 1610, 1551, 1530, 1486, 1461, 1430, 1350, 1302, 1237, 1173, 1074, 993, 917, 745, 698, 634 cm−1. HRMS: Calcd for C24H20O2N2 [M + Na]+ 391.1417; found 391.1418.
3.1.11. N-benzyl-3-hydroxy-3-(5-methoxy-indol-3-yl)indolin-2-one (12c)
White solid; mp: 204˚C - 206˚C. 1H NMR (400 MHz, DMSO-d6): δ 10.89 (s, 1H), 7.30 - 7.23 (m, 8H), 7.05 - 7.02 (m, 2H), 6.97 (d, J = 7.6 Hz, 1H), 6.70 - 6.67 (m, 2H), 6.55 (s, 1H), 4.91 (dd, J = 15.6 Hz, J = 22 Hz, 2H), 3.49 (s, 3 H). 13C NMR (100 MHz, DMSO-d6): δ 176.8, 152.7, 142.2, 136.4, 132.7, 131.9, 129.1, 128.5, 127.3, 125.2, 124.7, 124.3, 122.5, 114.7, 112.1, 111.2, 109.1, 102.2, 74.7, 54.9, 42.6. IR (KBr): υ 3354, 3283, 3047, 1702, 1611, 1582, 1527, 1486, 1461, 1350, 1306, 1211, 1174, 1065, 995, 931, 902, 854, 751, 698, 673 cm−1. HRMS: Calcd for C24H20O3N2 [M + Na]+ 407.1372; found 407.1357.
3.1.12. N-propargyl-3-hydroxy-3-(1-methyl-indol-3-yl)indolin-2-one (13c)
White solid; mp: 158˚C - 162˚C. 1H NMR (400 MHz, DMSO-d6): δ 7.41 - 7.33 (m, 4H), 7.19 (d, J = 8 Hz, 1H), 7.13 - 7.08 (m, 3H), 6.91 (t, J = 7.2 Hz, 1H), 6.60 (s, 1H), 4.56 (ABq, J = 16.4 Hz, J = 33.2 Hz, 2 H), 3.72 (s, 3 H), 3.31 (s, 1H). 13C NMR (100 MHz, DMSO-d6): δ 175.7, 141.2, 137.2, 132.5, 129.2, 127.9, 125.1, 124.6, 122.8, 121.3, 120.6, 118.7, 114.2, 109.7, 109.3, 78.0, 74.5, 32.4, 28.8. IR (KBr): υ 3322, 3254, 1711, 1609, 1535, 1463, 1426, 1369, 1335, 1244, 1213, 1170, 1110, 1071, 997, 934,892, 744, 671, 628. HRMS: Calcd for C20H16O2N2 [M + Na]+ 339.1109; found 339.1097.
3.1.13. N-propargyl-3-hydroxy-3-(5-methoxy-indol-3-yl)indolin-2-one (14c)
White solid; mp: 181˚C - 184˚C. 1H NMR (400 MHz, DMSO-d6): δ 10.9 (s, 1H), 7.41 (t, J = 7.6 Hz, 1H), 7.33 (d, J = 7.6 Hz, 1H), 7.25 - 7.18 (m, 2H), 7.12 - 7.07 (m, 2H), 6.75 (s, 1H), 6.70 (d, J = 8.4 Hz, 1H), 6.55 (s, 1H), 4.57 (ABq, J = 18 Hz, J = 45.2 Hz, 2H). 13C NMR (100 MHz, DMSO-d6): δ175.8, 152.9, 141.3, 132.5, 131.9, 129.1, 125.1, 124.6, 124.3, 122.8, 114.5, 112.1, 111.3, 109.2, 102.0, 78.1, 74.7, 74.5, 55.2 28.8. IR (KBr): υ 3411, 3325, 3276, 3089, 3052, 1705, 1611, 1583, 1530, 1486, 1463, 1438, 1360, 1291, 1242, 1209, 1177, 1111, 1059, 991, 924, 894, 835, 752, 702, 667 cm−1. HRMS: Calc. for C20H16O3N2 [M + Na]+ Calcd 355.1059; found 355.1047.
3.1.14. N-methyl-3-hydroxy-3-(5-methoxy-indol-3-yl)indolin-2-one (15c)
White solid; mp: 102˚C - 104˚C. 1H NMR (400 MHz, DMSO-d6): δ 10.85 (s, 1H), 7.39 - 7.31 (m, 2H), 7.23 (d, J = 8.8 Hz, 1H), 7.09 - 7.06 (m, 2H), 7.01 (s, 1H), 6.81 (s, 1H), 6.70 (d, J = 8.8Hz, 1H), 6.40 (s, 1H), 3.62 (s, 3H), 3.16 (s, 3H). 13C NMR (100 MHz, DMSO-d6): δ176.6, 152.7, 143.1, 132.7, 131.9, 129.2, 125.3, 124.4, 124.2, 122.4, 114.7, 112.1, 111.0, 108.4, 102.3, 74.7, 55.1, 25.9. IR (KBr): υ 3292, 3060, 1700, 1611, 1466, 1346, 1301, 1212, 1172, 1066, 997, 934, 903, 848, 752, 695, 668, 635 cm−1. HRMS: Calcd for C18H16O2N2 [M + Na]+ 331.1053; found 315.1064.
3.2. General Procedure for the Synthesis of Unsymmetrical 3,3Di(indolyl)indolin-2-ones (1e to 5e)
A mixture of 3-indolyl-3-hydroxy oxindoles (0.3400 mmol, 100 mg) and indole (0.4100 mmol, 48 mg) was added to a microwave-oven reaction vial, and then irradiated for 10 minutes at 150˚C. Then the residue as washed with 2 mL of EtOAc and mixed with silicagel and then evaporated the solvent under reduced pressure, and the mixture was purified by column chromatography using EtOAc in hexanes.
3.2.1. 3-(5-Methoxy-1H-indol-3-yl)-3-(1H-indol-3-yl)indolin-2-one (1e)
White solid; mp: 280˚C - 282˚C. 1H NMR (400 MHz, DMSO-d6): δ 10.95 (s, 1H), 10.79 (s, 1H), 10.59 (s, 1H), 7.35 (d, J = 8 Hz, 1H), 7.25 - 7.22 (m, 4H), 7.03 - 6.98 (m, 2H), 6.93 (t, J = 7.2 Hz, 1H), 6.89 - 6.81 (m, 3H), 6.70 - 6.67 (m, 2H), 3.51 (s, 3H). 13C NMR (100 MHz, DMSO-d6): δ 178.7, 152.4, 141.3, 136.9, 134.5, 132.1, 127.8, 126.0, 125.7, 125.0, 124.9, 124.3, 124.2, 121.4, 120.9, 120.7, 118.2, 112.0, 111.5, 110.4, 109.5, 103.3, 55.1, 52.5. IR (KBr): υ 3371, 1737, 1676, 1620, 1577, 1472, 1418, 1372, 1339, 1291, 1208, 1174, 1129, 1101, 1053, 1015, 958, 930, 889, 837, 796, 746, 679, 637 cm−1. HRMS: Calcd for C25H19O2N3 [M + Na]+ 416.1369; found 416.1383.
3.2.2. 3-1-Methyl(5-methoxy-1H-indol-3-yl)-3-(1H-indol-3-yl)indolin-2-one (2e)
White solid; mp: 280˚C - 282˚C. 1H NMR (400 MHz, DMSO-d6): δ 10.97 (s, 1H), 10.82 (s, 1H), 7.37 - 7.32 (m, 2H), 7.28 - 7.23 (m, 2H), 7.19 - 7.16 (m, 2H), 7.04 - 7.00 (m, 2H), 6.89 (d, J = 2.4 Hz, 1 H), 6.82 - 6.77 (m, 2H), 6.69 (dd, J = 2.4 Hz, J = 8.8 Hz, 1H), 6.58 (d, J = 2.4 Hz, 1H), 3.50 (s, 3H), 3.26 (s, 3H). 13C NMR (100 MHz, DMSO-d6): δ 177.0, 152.5, 142.8, 137.0, 133.7, 132.2, 128.1, 126.0, 125.7, 125.1, 124.7, 124.5, 122.3, 121.0, 120.7, 118.4, 113.9, 113.6, 112.2, 111.7, 110.6, 108.6, 103.0, 55.1, 52.2, 26.3. IR (KBr): υ 3396, 3345, 3314, 2924, 2854, 2363, 2337, 1734, 1692, 1608, 1462, 1423, 1350, 1292, 1212, 1172, 1123, 1086, 1038, 1016, 913, 891, 844, 795, 743, 692, 650 cm−1. HRMS: Calcd for C26H21O2N3 [M + Na]+ 430.1531; found 430.1538.
3.2.3. 3-1-Allyl(5-methoxy-1H-indol-3-yl)-3-(1H-indol-3-yl)indolin-2-one (3e)
White solid; mp: 266 - 268. 1H NMR (400 MHz, DMSO-d6): δ 10.99 (s, 1H), 10.83 (s, 1H), 7.36 (d, J = 8.4 Hz, 1 H), 7.33 - 7.29 (m, 2H), 7.24 (d, J = 8.4 Hz, 1H), 7.20 (d, J = 8 Hz, 1H), 7.11 (d, J = 7.6 Hz, 1H), 7.02 (t, J = 7.6 Hz, J = 14.8 Hz, 2H), 6.89 (d, J = 2.4 Hz, 1H), 6.82 (d, J = 2.8 Hz, 1H), 6.79 (d, J = 7.2 Hz, 1H), 6.68 (dd, J = 2.4 Hz, J = 8.4 Hz, 1H), 6.55 (d, J = 2.4 Hz, 1H), 5.95 - 5.86 (m, 1H), 5.21 (d, J = 1.6 Hz, 1H), 5.18 - 5.15 (m, 1H), 4.43 (d, J = 4.8 Hz, 2H), 3.48 (s, 3H). 13C NMR (100 MHz, DMSO-d6): δ 176.8, 152.6, 141.8, 137.0, 133.8, 132.2, 128.0, 126.0, 125.7, 125.2, 124.8, 124.4, 122.3, 121.1, 120.8, 118.4, 117.2, 114.0, 113.6, 112.3, 111.8, 110.8, 109.3, 102.9, 55.1, 52.2, 41.8. IR (KBr): υ 3754, 3708, 3345, 3010, 1736, 1669, 1606, 1540, 1478, 1456, 1358, 1293, 1208, 1171, 1093, 1018, 926, 841, 798, 744, 702, 632 cm−1. HRMS: Calcd for C28H23O2N3 [M + Na]+ 456.1688; found 456.1701.
3.2.4. 3-1-Benzyl(5-methoxy-1H-indol-3-yl)-3-(1H-Indol-3-yl)indolin-2-one (4e)
White solid; mp: 260˚C - 262˚C. 1H NMR (400 MHz, DMSO-d6): δ 11.00 (s, 1H), 10.9 (s, 1H), 7.38 - 7.37 (m, 3H), 7.32 - 7.25 (m, 6H), 7.15 - 7.09 (m, 2H), 7.04 - 6.98 (m, 2H), 6.93 (d, J = 2 Hz, 1H), 6.85 (d, J = 2.4 Hz, 1H), 6.75 (t, J = 7.6 Hz, J = 15.2 Hz, 1H), 6.69 (dd, J = 2 Hz, J = 8.8 Hz, 1H), 6.56 (d, J = 2 Hz, 1H), 5.02 (s, 2H), 3.42 (s, 3H).13C NMR (100 MHz, DMSO-d6): δ 177.2, 152.5, 141.8, 137.0, 133.8, 132.2, 128.6, 127.9, 127.5, 126.0, 125.6, 125.2, 124.9, 124.5, 122.4, 121.1, 120.7, 118.3, 113.9, 113.4, 112.2, 111.7, 110.8, 109.3, 103.0, 55.0, 52.3, 43.0. IR (KBr): υ 3347, 3025, 2850, 1699, 1676, 1607, 1535, 1483, 1458, 1358, 1291, 1209, 1172, 1133, 1099, 1016, 930, 847, 799, 745, 698, 63 cm−1. HRMS: Calcd for C32H25O2N3 [M + Na]+ 506.1839 found 506.1848.
3.2.5. 3-1-(Prop-2-ynyl)(5-methoxy-1H-indol-3-yl)-3-(1H-indol-3-yl)indolin-2-one (5e)
White solid; mp: 266˚C - 268˚C. 1H NMR (400 MHz, DMSO-d6): δ 10.99 (s, 1H), 10.85 (s, 1H), 7.38 - 7.36 (m, 2H), 7.27 - 7.25 (m, 4H), 7.08 - 7.01 (m, 2H), 6.87 (m, 1H), 6.81 - 6.79 (m, 2H), 6.71 - 6.69 (m, 1H), 6.63 (s, 1H), 4.65 (s, 2H), 3.51 (s, 3H), 3.31 (s, 1H). 13C NMR (100 MHz, DMSO-d6): δ 176.3, 152.6, 140.9, 137.0, 133.6, 132.2, 128.0, 125.9, 125.6, 125.3, 124.9, 124.6, 122.7, 121.2, 120.9, 118.4, 113.8, 113.2, 112.3, 111.7, 111.0, 109.4, 103.0, 78.2, 74.5, 55.2, 52.3, 29.0. IR (KBr): υ 3388, 3326, 3267, 1689, 1605, 1580, 1482, 1460, 1427, 1378, 1356, 1338, 1295, 1250, 1209, 1173, 1128, 1100, 1037, 1013, 932, 911, 888, 857, 800, 748, 703, 660 cm−1. HRMS: Calcd for C28H21O2N3 [M + Na]+ 454.1531; found 454.1538.
4. Conclusion
A simple and green alternative protocol for the synthesis 3-indolyl-3-hydroxy oxindoles in moderate to excellent yields is reported here. The unsymmetrical 3,3-di(indolyl)indolin-2-ones are also obtained in moderate to good yields under microwave irradiation. The highlights of the method are that no solvent, no catalysts are needed, and the reaction times are very short, i.e. five minutes for 3-indolyl-3-hydroxy oxindoles and ten minutes for unsymmetrical 3,3-di(indolyl)indolin-2-ones. Hence, this methodology can be conveniently used to synthesize the hybrid molecules of isatin and indoles in a short reaction time.
Acknowledgements
We thank the Department of Biotechnology and IIT-Madras for infrastructure.
NOTES
*Corresponding author.