Antioxidant, Enzyme Inhibitory and Anti-Obesity Potential of Sorrel Calyx Extracts in 3T3-L1 Adipocytes ()
1. Introduction
Obesity has become one of the greatest threats to global health with studies showing a strong association between obesity and increased risk of developing metabolic disorders such as cardiovascular diseases, hypertension, and diabetes [1] . Obesity is characterized by an increase in the level of lipid stores, changes in systemic energy and adipocyte metabolism [2] . The dramatic increase of obesity epidemic in the recent past is as a result of excess adipose tissue resulting from both hypertrophy and hyperplasia of adipocyte cells [3] . Hyperplasia results in the formation of new adipocytes from precursor cells, the preadipocytes while hypertrophy, leads to increase in lipid accumulation in the adipocytes [4] . In recent years, the exploration of natural compounds for the treatment of obesity and development of safe anti-obesity drugs has dramatically increased [5] . Natural bioactive components such as plant extracts have been found to contain medicinal properties such as potential for inducing apoptosis and inhibiting adipogenesis in adipocyte cells [6] .
Development of obesity is linked with complications in lipid metabolism and oxidative stress. A novel strategy currently being explored in the treatment of obesity is the development of inhibitors of nutrient digestion and absorption, with the aim of reducing energy intake through gastrointestinal mechanisms [7] [8] . The enzymes involved in lipid metabolic pathways represent a rich pool of potential therapeutic targets for obesity and other metabolic disorders [9] . Dietary lipids are the major sources of excess calories, and therefore, inhibition of triglyceride (TG) digestion provides a new strategy for the reduction of fat absorption [10] [11] . Pancreatic lipase (PL) is the major lipid digesting enzyme that plays a key role in the efficient digestion of triglycerides and is also responsible for hydrolysis of 50% - 70% of total dietary fats [7] . PL inhibition is one of the most widely studied mechanisms for treatment of obesity [12] .
High consumption of carbohydrate is another factor for increased fat deposition because excess carbohydrates are converted into fats in the body. Natural bioactive compounds shown to inhibit carbohydrate hydrolyzing enzymes such as α-glucosidase have been recognized as potential therapeutic targets for modulation of postprandial hyperglycemia in type 2 diabetes mellitus patients [13] [14] . Inhibitors of α-glucosidase enzyme can reduce uptake of dietary carbohydrate, suppress postprandial hyperglycemia, and therefore, may be useful in treating patients with diabetes and/or obesity [15] . A number of α-glucosidase inhibitors such as acarbose and voglibose interfere with the activity of carbohydrate-digesting enzymes delaying glucose absorption [16] .
There has been considerable interest in the investigation of the anti-obesity effects of natural products. Existing evidence has shown that phytochemicals exert anti-obesity effects through regulation of various metabolic pathways, including lipid absorption, the intake and expenditure of energy, the increase of lipolysis, and the decrease in proliferation of adipocyte cells [17] . Hibiscus sabdariffa (sorrel), is a member of the Malvaceae family and an annual tropical plant native to India and Malaysia [18] . Hibiscus sabdariffa L. calyx extracts have been used as traditional medicine against hypertension, pyrexia, inflammation, and cancer due to the presence of various bioactive components [19] . Sorrel extracts contain various phytochemicals that may have inhibitory effects on adipocyte differentiation and therefore the objective of this study was to study anti-obesity effects of sorrel calyx extracts on 3T3-L1 adipocyte cells.
2. Materials and Methods
2.1. Preparation of Sorrel Extracts
Dried sorrel calyces were purchased from a local health store (Huntsville AL, USA). They were ground to fine powder using an electric blender (Laboratory blender, Warring commercial, Torrington, CT, USA). Phytochemical extracts were prepared in 80% methanol and hot water. Five grams of sample was extracted in 100 ml of 80% me- thanol and concentrated in rotary evaporator (Safe aire, Fisher Hamilton, Gaithersburg, MD, USA). For water extraction, 20 g of sample was extracted with 100 ml of water at 100˚C for one hour and concentrated. The concentrates were stored at −20˚C until further analysis [20] .
2.2. Determination of Total Phenolics, Flavonoids and Anthocyanins
The total phenolic content was determined according to the method of Singleton et al. (1999) [21] with modifications. Briefly, Sorrel extracts (12.5 μl) were oxidized with 12.5 µL of Folin Ciocalteau reagent and incubated for 5 minutes, neutralized by 125 µl of 7% NaCO3 at room temperature for 90 minutes. Absorbance was read at 750 nm using a microplate reader (Synergy HT, Bio Tek Instruments, Winooski, VT, USA). The total phenolic content was expressed as mg GAE/100g dry weight.
The total flavonoid content was determined according to the method of Kim et al. (2003) [22] . Appropriate dilutions of sorrel samples were mixed with 7.5 µL of 5% NaNO2 solution and 15 µL of 10% AlCl3. The mixture was incubated at room temperature for 5 minutes before adding 50 µL of 1 M NaOH and 40 μL of distilled water. The absorbance was read at 510 nm in microplate reader.
The total anthocyanins content was determined in duplicate using the pH differential method [23] . Absorbance was read at 520 nm and 700 nm in buffers at pH 1.0 and 4.5, using a microplate reader (Synergy HT, Bio Tek Instruments, Winooski, VT, USA). The concentration was determined using monomeric anthocyanin pigment
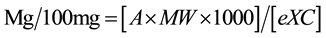
where A= (A520 nm - A700 nm)pH 1.0―(A520 nm - A700 nm)pH 4.5; MW is the molecular weight of cyanidin-3-glucoside (449.2 g/mol), C―concentration of sample; eX, is the molar extinction coefficient―26,900.
2.3. Determination of 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) Radical-Scavenging Activity
Free radical scavenging activity was determined by following modified method of Brand-Williams, Cuvelier & Berset (1995) [24] . Briefly, 40 µL of appropriately diluted concentrations of sorrel extracts were added to 200 µL of 0.1 mM 2,2-Diphenyl-1-picrylhydrazyl (DPPH) in methanol. Absorbance was measured at 517 nm at 90 minutes after incubating at room temperature (Synergy HT, Bio Tek Instruments, Winooski, VT, USA).
2.4. Determination of Ferric Reducing Antioxidant Power (FRAP)
The FRAP assay was performed according to Benzie & Strain (1999) [25] . Briefly, 10 µL of known dilutions of sorrel extracts were mixed with 200 µL of FRAP reagent and incubated for 10 minutes at 37˚C. Change in absorbance was measured at 593 nm (Synergy HT, Bio Tek Instruments, Winooski, VT, USA) and the results are expressed as mmol Fe2+/g dry weight.
2.5. Enzyme Inhibition Assays
The inhibition of pancreatic lipase (PL) (in-vitro) was determined using p-nitrophenyl butyrate (p-NPB) as a substrate [26] . A stock solution (1mg/1ml) of PL was prepared in 0.1 mM potassium phosphate buffer (pH 6.0). SME and SWE at different concentrations were pre-incubated with enzyme for 1 hour in a potassium phosphate buffer (0.1 mM, pH 7.2, combined with 0.1 ml Tween 80). Subsequently, 5 µl of substrate (25 mM pNB in Dimethylformamide (DMF) was added and incubated for 5 minutes at 30˚C. The amount of 2, 4-dinitrophenol released in the reaction was measured at 360nm in microplate reader (Synergy HT, Bio Tek Instruments, Winooski, VT, USA).
Various concentrations (0.2 - 4 mg/ml) of SWE and SME, and 50 µl of 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M sodium chloride) containing α-amylase solution (4 units/ml) were incubated at 25˚C for 10 minutes. After pre-incubation, 50 µl of 1% starch solution was added and incubated for another 10 min. Reaction was terminated by the addition of 100 µl of dinitrosalicylic acid color reagent on steam for 5 minutes. After cooling room temperature, mixture was diluted with distilled water and absorbance read at 540 nm (Synergy HT, Bio Tek Instruments, Winooski, VT, USA).
The α-glucosidase enzyme inhibitory assay was performed in a 96 well plate as described by kim et al., 2010 [27] with slight modifications. A 50 µl of various concentrations of SME and SWE, and 100 µl of containing α-glucosidase solution (1.0 U/ml in 0.1 M phosphate buffer (pH 6.9)) were incubated at 25˚C for 10 min. After pre-incubation, 50 µl of 5 mM p-nitrophenyl-α-D-glucopyranoside was added to each well and incubated at for 5 min and absorbance was read at 405 nm (Synergy HT, Bio Tek Instruments, USA).
Enzyme inhibitory activity was calculated as follows:
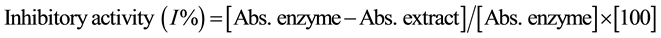
2.6. Cell Culture and Differentiation
3T3-L1 mouse embryo fibroblasts were obtained from American Type Culture Collection (Manassas, VA, USA). Preadipocyte cells were grown in Dulbecco's Modified Eagle Medium (DMEM) containing 10% calf bovine serum (CBS) and 1 mM sodium pyruvate. For standard adipocyte differentiation, cells were treated with 10% fetal bovine serum (FBS), 10 µg/mL insulin, 1 µM dexamethasone, and 0.5 µM 3-isobutyl-1-methyl-xanthine for 48 hours. The cells were then maintained in a maintenance media containing 10% FBS and 10 µg/ml insulin for additional 4 - 6 days. Various concentrations (100 - 1000 µg/ml) of SME and SWE were added to media at differentiation and maintenance stages of cells to evaluate the anti-obesity properties. Cells that were not treated with extracts served as control.
2.7. Cell Viability Assay
Cell respiration as an indicator of cell viability and proliferation was determined using a mitochondrial-depen- dent reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) to formazan using a commercial assay kit (Abnova, Walnut, CA, US) according to the manufacturer’s instructions and absorbance read at 490 nm in a plate reader (Bio-Tek Synergy HT, Winooski, VT, US) to determine the concentration of formazan which is proportional to the number of live cells
2.8. Oil Red O Staining and Determination of Lipid Content
Cells were stained with Oil Red O (ORO) as described by Suryawan & Hu (1993) [28] . Culture dishes were washed with PBS, fixed with 10% formalinand stained with a filtered ORO solution (0.5 grams of ORO in 100 mL of isopropyl alcohol) for 30 minutes. Cells were washed twice and visualized. Digital images were obtained by Nikon CT 120 microscope and photographs were taken by SPOT 2.0 digital camera. Stained cells were air dried, dissolved in isopropanol and absorbance was measured at 520 nm [27] .
2.9. Lipolysis, Triglyceride Content and Glycerol-3-Phosphate Dehydrogenase (GPDH) Activity
Glycerol release was used to assess the lipolytic effect of sorrel extracts on 3T3-L1 adipocyte cells according to the glycerol cell-based assay kit (Abnova, Walnut, CA, USA). The glycerol was quantified at an absorbance of 540 nm in a microplate reader (Bio-Tek Synergy HT, Winooski, VT, US). The amount of glycerol released was calculated by the equation of glycerol standard curve. Cellular TG contents were measured using a commercial TG assay kit (Abnova, Walnut, CA, USA). The GPDH assay was performed using a spectrophotometric method for determination of the disappearance of β-nicotinamide adenine dinucleotide during GPDH-catalyzed reduction of dihydroxyacetone-3-phosphate using a commercial GPDH activity assay kit (Clontech labs, Mountain View, CA, USA).
2.10. Apoptosis Assay
A Cellular DNA Fragmentation kit was used (Roche, Indianapolis, IN, US) to determine extent of apoptosis. Cells grown in 96-well plates, were treated with sorrel extracts for 24 hours, and assayed as per the manufacturer’s instructions.
2.11. Statistical Analysis
All experiments were performed in triplicates. Results are presented as means ± SEM. ANOVA was used to determine any significant differences among the treatment groups. The means were separated using Tukey’s Studentized Range Test at P < 0.05 (SAS 9.1).
3. Results
3.1. Phytochemical Content Antioxidant Activity of Sorrel Calyx Extracts
Phytochemical content in extracts were varied between types of extractions (Table 1). Water extraction of sorrel yielded higher (p < 0.05) total phenolic content (317.27 mg GAE/100g) compared to SME (158.31 mg GAE/ 100g). However, method of extraction did not show significant differences in flavonoid content. Total flavonoid content determined as catechin equivalents was higher (100.08 mg CE/100g) in SWE. However, anthocyanin content was eight times higher in SME compared to SWE (88.77 mg/100mg).
![]()
Table 1. Polyphenols, flavonoid and anthocyanin contents of sorrel methanol and water extracts.
SME-sorrel methanol extracts; SWE-sorrel water extracts; GAE: Gallic Acid Equivalents; CE: Catechin Equivalents. Values are Mean ±SD; n = 3. abValues not sharing common superscript are significantly different (p < 0.05) using Tukey’s studentized range test.
Antioxidant potential analyzed as DPPH radical scavenging activity and ferric reducing potential were shown in Table 2. Highest antioxidative activity was found in SWE. DPPH radical inhibition was increased from 8% - 72% with an increase in concentration of SWE from 0.02 to 0.83 mg/ml. More reduction in DPPH radical was observed in SWE (IC50-0.33 mg/ml) compared to SME (IC50-0.82 mg/ml). Ferric reducing potential of SWE extracts was 1.3 times higher than that of SME.
3.2. Anti-Lipase Activity of Sorrel Calyx Extracts
The ability of sorrel extracts to inhibit pancreatic lipase activity in-vitro was investigated and results are shown in Figure 1. Anti-lipase activity was found to be similar in both extracts. Inhibition of lipase activity (3% - 46%) was increased with increase in concentrations (0.05 - 4 mg/ml) of extracts. The percentage of lipase inhibition remained constant at concentrations of 1 - 4 mg/ml for both SME and SWE. Both extracts exhibited moderate (>38%) inhibition of lipase activity at 1 mg/ml.
3.3. α-Glucosidase and α-Amylase Inhibitory Activities
SME and SWE exhibited higher inhibition of both α-glucosidase (Figure 2) and α-amylase (Figure 3) activities in a concentration dependent manner. Incubation with SME resulted in a higher percent (78%) of inhibition of α-glucosidase activity at 4 mg/ml compared to SWE (42%). Both extracts exhibited similar inhibition (35%) of enzyme activity at concentrations ranging from 0.2 - 0.75 mg/ml. Inhibitory potential of α-glucosidase enzyme by SWE remained constant (30% - 40%) after 1 mg/ml. A higher inhibition of α-amylase activity was seen with an increase of SME concentrations ranging from 0.2 - 2 mg/ml. However, there was a minimal increase in inhibition of α-amylase with concentrations of SME ranging from 2 - 4 mg/ml. SWE exhibited a lower percentage (38.8%) of inhibition of α-amylase activity at 4 mg/ml compared to SME (52.3%) at the same concentration.
3.4. Effects of Sorrel Extracts on Lipid Accumulation
Accumulation of excess energy as triglycerides in adipocytes was analyzed quantitatively and qualitatively with Oil Red O staining. The cells were treated with different concentrations (200 - 1000 µg/ml) at the differentiation and maintenance stages. The Oil Red O staining of cells revealed that SME and SWE suppressed lipid accumulation, by showing less accumulation of lipid droplets (Figure 4). The highest lipid accumulation was observed in untreated differentiated cells indicating excess storage cytoplasmic triglycerides (Figure 4(b)) after 6 days. Treatment of 3T3-L1 adipocyte cells with 200 µg/ml and 1000 µg/ml of SME and SWE decreased lipid accumulation and led to fewer lipid accumulated cells as shown in Figures 4(c)-(f) respectively. Quantitative analysis of Oil Red O staining exhibited an inhibition of lipid accumulation by sorrel extracts in a concentration-de- pendent manner (Figure 5). Significant (p < 0.05) reduction in lipid accumulation at differentiation stage was observed in cells treated with SME at 400 µg/ ml. Whereas; SWE resulted in significant reductions in lipid content at 600 µg/ml at differentiation stage compared to control untreated adipocytes. Incubation with extracts resulted in a significant reduction in lipid accumulation at concentrations above 800 g/ml compared to untreated cells. Highest (p < 0.05) reduction (45%) in lipid content was seen in cells treated with SME at a concentration of 1000 µg/ml at the differentiation stage. After incubation with SME-1000 µg/ml at differentiation stage, lipid reduction was significantly higher in cells treated at both stages and SME at 800 µg/ml treated at both stages. The percent lipid content decreased from 98.2% - 63.7% with the highest reduction in cells treated with SME at a concentration of 1000 µg/ml at the maintenance stage. SME was more effective in reducing lipid accumulation in treated cells compared to SWE.
![]()
Figure 1. Inhibition of pancreatic lipase activity by sorrel methanol (SME) and water extracts (SWE).
![]()
Figure 2. Inhibition of α-glucosidase activity by sorrel methanol (SME) and water extracts (SWE).
![]()
Figure 3. Inhibition of α-amylase activity by sorrel methanol extract (SME) and sorrel water extract (SWE).
![]()
Figure 5. Relative lipid content post-treatment with sorrel methanol (SME) and water (SWE) extracts at differentiation stage (DS) and maintenance stage (MS). Values are Mean, n = 3. abBars not sharing common superscript are significantly different (p < 0.05) using Tukey’s studentized range test.
![]()
Table 2. DPPH and FRAP in sorrel methanol and water extracts.
FRAP: Ferric Reducing Antioxidant Power, DPPH: 1, 1-Diphenyl-2-picrylhydrazyl, SME: Sorrel methanol extracts, SWE: Sorrel water extracts. Values are Mean ± SD; n = 3. abValues not sharing common superscript are significantly different (p < 0.05) using Tukey’s studentized range test.
3.5. Triglyceride Content and Lipolysis
Exposure of 3T3-L1 cells to SME and SWE at the differentiation and maintenance stages resulted in a decrease in triglyceride content in a concentration dependent manner (Figure 6) compared to untreated adipocytes. A significant difference in triglyceride content was observed at all tested concentrations of extracts above 600 µg/ ml except SWE treated at 600 µg/ml at maintenance stage. Methanol extract (400 µg/ml) at differentiation stage cells also resulted in significantly lower triglyceride content compared to cells treated with no sorrel extracts. Treating extracts at different stages did not show any effect in reducing the triglyceride content in water extracts. However, significant differences in lipid content at stages of treatment were observed with SME at 600 & 800 µg/ml. No significant stage effect on triglyceride level was observed when cells were treated at the highest concentration. A consistent decrease in triglyceride content ranged from 6.08 to 2 mg/dl and 6.04 to 2.31 mg/dl in cells treated at the differentiation stage with SME and SWE respectively. Highest (p < 0.05) (35%) decrease in triglyceride content was seen in cells exposed to SME at the differentiation stage compared to the control. Lipolytic activity of sorrel extracts in adipose tissue was determined by using glycerol release as an indicator. At each concentration tested (200 - 1000 µg/ml), the exposure of 3T3-L1 adipocyte cells to SME and SWE at the differentiation and maintenance stages resulted in a similar release of glycerol (Figure 7). There was no stage difference found on glycerol release in adipocytes by treating sorrel extracts at all tested concentrations. Lower concentrations (200 & 400 µg/ml) of both extracts did not induced significant (p < 0.05) release of free glycerol except SME treated at 400 mg/ml differentiation stage which has significantly higher glycerol release compared untreated adipocytes. High (600 - 1000 µg/ml) concentrations of extracts at both stages induced higher (p < 0.05) glycerol release except SWE 600 mg/ml at maintenance stage. A tenfold increase in stimulation of lipolysis was seen in cells treated with 1000 µg/ml at the maintenance and differentiation stages with SWE compared to the control. A 75% glycerol release was seen in cells treated with SME at a concentration of 1000 µg/ml at the maintenance stage compared to the control. Highest (p < 0.05) lipolysis was observed in cells treated with 1000 µg/ml at both stages and cells treated with 800 mg/ml of SME at differentiation stage.
3.6. Effects of Sorrel Extracts on Cell Viability
Effect of sorrel extracts on cell viability (Figure 8) was determined by MTT assay. Treating with extracts for 8 days exhibited a moderate decrease in cell viability of adipocytes. No differences (p < 0.05) in survival of cells were found when cell were treated with 200 & 400 µg/ml of extracts at both stages compared to the control except SWE 400 µg/ml at differentiation stage. Incubating cells with high concentration (1000 µg/ml) of SWE and SME exhibited low (p < 0.05) viability (54.4% and 61.1%) of cells compared to the control. Reduction (p < 0.05) in viability of cell was observed at tested concentrations above 600 µg/ml. Methanol extracts (600 µg/ml and above) exhibited differences (p < 0.05) in viability of cells at different stages where cells treated with SME at differentiation stage exhibited lower viability compared to cells treated SME at maintenance stage. However, SWE did not show any effect on stage of treatment at the same tested concentrations. At the differentiation
![]()
Figure 6. Effects of sorrel methanol (SME) and water (SWE) extracts on triglyceride content in 3T3-L1 cells treated at differentiation stage (DS) and maintenance stage (MS).
![]()
Figure 7. Effects of sorrel methanol (SME) and water (SWE) extracts on 3T3-L1 adipocyte lipolysis treated at differentiation stage (DS) and maintenance stage (MS). Values are Mean, n = 3. abBars not sharing common superscript are significantly different (p < 0.05) using Tukey’s studentized range test.
![]()
Figure 8. Effects of sorrel methanol (SME) and water (SWE) extracts on 3T3-L1 adipocyte cells viability treated at differentiation stage (DS) and maintenance stage (MS). Values are Mean, n = 3. abBars not sharing common superscript are significantly different (p < 0.05) using Tukey’s studentized range test.
stage, incubating with SME exhibited low viability at 200 - 1000 µg/ml compared to SWE (8% - 39%) at the same concentrations.
3.7. Effect of Sorrel Extracts on Apoptosis of 3T3-L1 Adipocyte Cells
To determine induction of apoptosis by sorrel extracts, a photometric cellular DNA fragmentation assay was conducted. Treating cells with 1000 µg/ml of SME at the differentiation stage caused a two fold increase in apoptosis compared to the control (Figure 9). A 3% - 7% lower induction of apoptosis was seen in cells treated with 200 - 400 µg/ml of SME at the differentiation stage compared to untreated adipocytes. Higher (p < 0.05) apoptosis was observed in adipocytes treated with sorrel extracts on and above 800 µg/ml and also in SME 600 µg/ml at maintenance stage. Treatment with extracts at different stages did not yield any significant differences in apoptosis except at highest (1000 µg/ml) tested concentration where, both extracts treated at differentiation
![]()
Figure 9. Effects of sorrel methanol (SME) and water (SWE) extracts on apoptosis of 3T3-L1 adipocyte cells treated at differentiation stage (DS) and maintenance stage (MS). Values are Mean, n = 3. abBars not sharing common superscript are significantly different (p < 0.05) using Tukey’s studentized range test.
stage showed higher (p < 0.05) apoptosis compared to maintenance stage. At 800 µg/ml, a 35% increase in apoptosis was seen in cells treated with SME at the differentiation and maintenance stages compared to the control. A low but consistent increase in apoptosis was seen in cells treated with sorrel concentrations ranging from 600 to 1000 µg/ml. Highest apoptosis in adipocytes was recorded when cells were treated with SWE at differentiation stage (1000 µg/ml) followed by SWE and SME at the same concentration at maintenance stage.
3.8. Effect of Sorrel Extracts on Glycerol-3-Phosphate Dehydrogenase (GPDH) Activity
The glycerol-3-phosphate dehydrogenase (GPDH) assay was used as a marker of late adipocyte differentiation. Marginal differences were observed in GPDH activity in 3T3-L1 adipocyte cells treated with SME and SWE (200 - 1000 µg/ml) at the differentiation and maintenance stages compared to the untreated differentiated cells (Figure 10). A 15% - 17% decrease in GPDH activity was seen in adipocytes treated with 1000 µg/ml of SME and SWE at the differentiation stage. A similar GPDH activity was seen in 3T3-L1 adipocytes treated with concentrations of SME and SWE ranging from 200 to 800 µg/ml compared to the control. A significant reduction (p < 0.05) in GPDH activity was observed in cells treated with both extracts at both stages. Treating extracts at different stages did not show any effect in reducing GPDH activity.
4. Discussion
In the present study, we examined the anti-obesity effect of sorrel calyx extracts in 3T3-L1 cells by measuring anti-adipogenic effects and inhibitory activities of SME and SWE on obesity-related enzymes. The results showed that the solvent used in the extraction of phytochemicals affect the extractability of polyphenols present in sorrel. The higher phenolic and flavonoid contents observed in SWE compared to SME may be attributed to the high polarity and temperature of the hot water used as a solvent. A study by Prenesti et al. (2007) [29] reported similar findings on the polyphenolic contents of Hibiscus sabdariffa L. flowers extracted with water and ethanol. The higher phenolic content observed in water extracts may be attributed to the high temperature used in extraction which can accelerate the release of phenolic acids from conjugated esters, or their insoluble forms [30] .
Anthocyanin pigments have been previously reported as the major source of antioxidant activity in sorrel [31] . SME yielded eight-fold higher anthocyanin content compared to SWE. Two anthocyanin pigments; delphinidin-3-sambubioside and cyanidin-3-sambubioside have been previously identified as the key contributors to sorrel antioxidant activity [32] . Results suggest that using methanol as solvent, yielded more anthocyanins in sorrel extracts compared to water.
Reducing power is commonly used as an indicator of electron-donating activity, which is an important prop-
![]()
Figure 10. Effect of sorrel methanol (SME) and water (SWE) extracts on glycerol-3-phosphate (GPDH) activity treated at Differentiation stage (DS) and Maintenance stage (MS). Values are Mean, n = 3. abBars not sharing com- mon superscript are significantly different (p < 0.05) using Tukey’s studentized range test.
erty in testing for antioxidative potential of phytochemicals [30] . In the present study, higher inhibition of DPPH radical and FRAP were seen with SWE compared to SME. The amount of polyphenolic compounds present in a plant account for its antioxidant activity [33] . The higher anti-oxidative activity of SME compared to SWE might be due to the presence of a higher polyphenolic content compared to SME. The high FRAP of sorrel extracts may be attributed to the efficacy of the major compounds found in sorrel; chlorogenic acid and its derivatives which act as reductants [34] .
Inhibition of pancreatic lipase (PL) enzyme is one of the most widely studied mechanisms for determination of the potential efficacy of natural bioactive compounds as anti-obesity agents [5] . The inhibition of PL activity by SME and SWE was almost similar at all tested concentrations. The extracts of P. vulgaris showed PL inhibitory activity of 27.4% at a concentration of 25 µg/ml [35] . Our results suggest sorrel extracts have the potential to inhibit PL activity in a concentration-dependent manner. Also, the method of sorrel extractions did not have a significant effect in inhibition of PL activity.
Inhibition of enzymes involved in the hydrolysis of carbohydrates such as α-glucosidase and α-amylase have been exploited as a potential therapeutic approach for controlling postprandial hyper-glycemia and obesity [36] . In the present study, results showed that sorrel extracts inhibited α-glucosidase and α-amylase activities in a concentration-dependent manner (0.2 - 4 mg/ml). SME resulted in a higher percent (78%) inhibition of α-glu- cosidase activity at (4 mg/ml) compared to SWE (42%). Moreover, SME showed higher percent (52.3%) inhibition of α-amylase activity compared to 38.8% at the same concentration. Studies [37] [38] indicated that hibiscus acid and cyanidin-3-glucoside present in roselle are active pancreatic α-amylase inhibitors. Therefore, the higher inhibition of α-amylase activity observed with SME compared to SWE may be attributed to the higher extractability of the active pancreatic α-amylase inhibitors with a methanol solvent compared to hot water.
Adipocyte differentiation and lipid accumulation are associated with the occurrence and development of obesity [39] . In the present study, sorrel calyx extracts inhibited lipid accumulation in 3T3-L1 cells in a concentration-dependent manner. The treatment of 3T3-L1 preadipocyte cells with dietary flavonoids quercetin, kaempferol, and catechins resulted in high inhibition of adipogenesis [40] . Results from the present study suggest that both SME and SWE played a critical role in preventing adipogenesis and the accumulation of cytoplasmic lipid droplets in 3T3-L1 cells treated at the differentiation and maintenance stages.
Lipolysis in fully differentiated 3T3-L1 adipocyte cells was examined to determine whether sorrel calyx extracts reduce lipid content by increasing stimulation of glycerol release. During the differentiation stage, treatment of 3T3-L1 adipocyte cells with SME and SWE at a concentration of 1000 µg/ml induced a tenfold greater release of glycerol into the culture medium compared to the untreated differentiated cells. A previous study by Harmon & Harp (2001) [41] reported similar findings with treatment of 3T3-L1 cells with genistein and epinephrine. Cells treated with SME at the differentiation stage exhibited a higher glycerol release at all tested concentrations (200 - 1000 µg/ml) compared to cells treated at the maintenance stage. Results suggest that incubation period and the stage during the adipocyte life cycle when cells are exposed to sorrel extracts affected the amount of glycerol release. Based on the results, it is reasonable to suggest that lipolysis is one mechanism by which sorrel extracts may reduce adipose tissue mass by breaking down triacylglycerols in the 3T3-L1 adipocyte cells.
The breakdown of triacylglycerols in adipocytes and release of glycerol and fatty acids are important for the regulation of energy homeostasis [42] . In the present study, results showed a consistent decrease in triglyceride content ranging from 6.08 to 2 mg/dl and 6.04 to 2.31 mg/dl in cells treated at differentiation stage with SME and SWE respectively. The reduction in triglyceride content observed in 3T3-L1 cells may be attributed to the lipolytic activity of SME and SWE. As previously stated, SME and SWE induced lipolysis in a concentration- depend manner with the highest glycerol release by cells treated with SME and SWE seen at 114.01 nmol/ ml and 112.58 nmol/ ml compared to the control (10.12 nmol/ ml). Moreover, at 1000 µg/ml, the highest inhibition of triglyceride accumulation by SME and SWE was observed at 2.0 nmol/ ml and 2.31 nmol/ ml compared to the control (6.35 nmol/.ml). Therefore, it can be suggested that the reduction in triglyceride concentration seen in 3T3-L1 cells, may be caused by lipolytic effects of SME and SWE. In addition, a 15% to 17% decrease in glycerol-3-phosphate dehydrogenase (GPDH) activity was observed in cells treated with SME and SWE, which may be responsible for the lower triglyceride levels seen in 3T3-L1 adipocyte cells compared to the control.
A concentration-dependent decrease in viability was seen in 3T3-L1 adipocyte cells treated at the differentiation and maintenance stages. Percentage (95.5% - 53.4%) reduction in viability compared to the control was seen in 3T3-L1 adipocyte cells treated at the differentiation stage. Genistein at 200 µmol/l was shown to cause a 56% decrease in the viability of mature 3T3-L1 adipocytes [6] . Based on the results from the present study, it is reasonable to suggest that the effects observed on lipid accumulation, is due not only to decreased adipogenesis, but also to the effect of SME and SWE on cell viability.
The adipose tissue mass can be decreased by eliminating adipocytes through apoptosis (Kim et al., 2007). In the present study, exposure of 3T3-L1 cells to SME and SWE during the differentiation stage for a period of 6 days, showed a concentration-dependent induction of apoptosis. At lower concentrations (200 - 600 µg/ml), sorrel extracts did not show apoptotic effects. However, an approximately two-fold increase in induction of apoptosis was seen in cells treated with 1000 µg/ml of SME at the differentiation stage. Results suggest that sorrel extracts may be used to decrease adipose tissue mass through apoptosis.
5. Conclusion
Hibiscus sabdariffa L. (sorrel) contains bioactive compounds that are important in modulating obesity through anti-oxidant related mechanisms and inhibition of adipogenesis in 3T3-L1 adipocyte cells. The inhibition of pancreatic lipase, α-glucosidase, and α-amylase activities (in-vitro) by SME and SWE may provide a potential means of developing safe therapeutic approaches of preventing and/or treating obesity. The inhibition of lipid accumulation and intracellular triglyceride accumulation in 3T3-L1 adipocyte cells by SME and SWE may suggest that sorrel extracts play a critical role in preventing adipogenesis and reducing accumulation of cytoplasmic lipid droplets. This is important in preventing development of obesity by decreasing hyperplasia and hypertrophy of white adipocyte cells. Based on the results, treatment of 3T3-L1 cells with SME and SWE stimulate lipolysis. The induction of apoptosis in 3T3-L1 cells by SME and SWE was low, suggesting that other mechanisms might be responsible for the decrease in number of 3T3-L1 adipocyte cells.
Acknowledgements
Funding was provided by the Alabama Agricultural Experiment Research Station, USDA Evans Allen Grant.