Field Efficacy of Chemical Pesticides against Maruca vitrata Fabricius (Lepidoptera: Crambidae) Infesting Soybean in Brazil ()
1. Introduction
The legume pod borer Maruca vitrata Fabricius (Lepidoptera: Crambidae; Syn: Maruca testulalis), is distributed through the tropical and subtropical regions of the world [1] . M. vitrata is a serious pest of grain legumes because of its extensive host range, destructiveness, and distribution [2] [3] .
The larval stages of M. vitrata are destructive within agricultural and forest eco-systems as they feed on flowers and pods of more than 39 host plants, including two non-leguminous hosts [4] [5] . Host plants are mainly species from the Family Fabaceae (leguminous plants) [6] [7] . M. vitrata attacks Vigna unguiculata subsp. unguiculata (cowpea), Vigna unguiculata subsp. sesquipedalis (yardlong bean), V. radiata (mung bean), Glycine max (soybean), Pueraria phaseoloids (puero), Phaseolus lunatus (lima bean), and Cajanus cajan (pigeonpea) and often causes significant yield losses in sub-Saharan Africa [2] [8] , Southeast Asia [9] [10] , South Asia [11] [12] and central and South America [13] .
In Brazil, M. vitrata is considered as a seasonal pest on soybean. The attack of this lepidopteran occurs mainly for weather conditions, where low relative humidity and high temperatures are favorable for its occurrence on soybean fields [14] .
Larvae are yellow to light brown shiny, with dark spots, bristles distributed in the body, and well-defined body segmentation. This pest can attack pods, armpits, stems, and petioles of soybean, and eventually damage inflorescences, with habits and similar damage to the bean shoot borer Epinotia aporema (Lepidoptera: Tortricidae) [15] .
M. vitrata larvae feed on flowers, buds, and pods by webbing them. This typical feeding habit protects the larvae from natural enemies and other adverse factors, including insecticides. Moths prefer to oviposit at the flower bud stage. Larvae move from one flower to another, and each may consume 4 - 6 flowers before larval development is completed. Third- to fifth-instar larvae are capable of boring into the pods, and occasionally into peduncle and stems [2] . Moths and larvae are nocturnal [16] . Infestation is highest in flowers, flower buds, terminal shoots and pods respectively. Karel [17] also observed more larvae (52.3%) on flowers than on pods (37.8%), and leaves (9.9%).
The damage caused by this pest are usually difficult to detect, but can cause plants breakdown by wind action. Its field observation can be made by pods inspection or by performing longitudinal cuts in the stem of plants attacked. In addition, the entrance orifice on pods and petioles of soybean plants is not blocked by your stool.
Control of M. vitrata damage to crops largely relies upon the timely application and availability of chemical insecticides [8] , but their effectiveness is hindered by the tight larval webbing that reduces pesticide exposure [2] . Furthermore, the cost of insecticides is prohibitive to most subsistence farmers in developing nations [18] - [20] . The losses and subsequent control challenges posed by M. vitrata have led to the emergence of this species as a major threat to economic and humanitarian well-being in developing and under-developed nations.
Furthermore, control of M. vitrata damage to field crops depends mainly on chemical insecticide applications [8] , but success is variable due in part to 1) the web structures larvae construct which shields them from insecticide sprays [2] ; 2) the evolution of resistance to insecticides [10] [21] ; and 3) the cost of chemical sprays in developing nations [19] [20] .
Due to the lack of chemical control studies, the aim of this study was to evaluate the field efficacy of chemical pesticides when spray applied on soybean against M. vitrata.
2. Material and Methods
2.1. Study Location
The experiment was conducted on an experimental area of Fundação MS, in Maracaju, MS, Brazil, in the growing season 2012/13. The Fundação MS is located at latitude 21˚36'52" South, longitude 55˚10'06" West and altitude 384 m.
2.2. Description of Sampling Area and Experimental Design
The experiment was arranged in a randomized block design with six treatments (teflubenzuron, flubendiamide, methomyl, chlorantraniliprole + lambda-cyhalothrin, chlorpyrifos, and a control treatment without insecticide) and five replications (Table 1). The soybean cultivar used was BMX Turbo RR.
Sowing was done mechanically on October 23, 2012 with an average density of 15 seeds per meter. The soil
![]()
Table 1. Treatments (active ingredient, chemical group, and dose) used to evaluate the field efficacy of chemical insecticides against Maruca vitrata. Maracaju, MS, 2013.
was prepared on a direct seeding system and corrected with 380 kg・ha−1 of 02-20-20 (N-P-K). 36 plots were outlined, each of which consisted of seven 7-m rows, spaced 0.45 cm apart. The three central plant rows per plot were evaluated, excluding one meter from either end of the rows.
Insecticide application was realized on December 20, 2012, at 06:30 am, using a pressurized sprayer (CO2), equipped with a six-nozzle bar type TJ 06 11002, 0.5 m apart and calibrated of 160 L・ha−1. Conditions during insecticide application were 23.2˚C, 80% relative humidity, total wind absence, and soybean was on R3 stage.
2.3. Sampling Methods
Evaluations were performed at one, four, seven, 10, and 14 days after pesticides application (DAA), and were based on the percentage of attacked plants with M. vitrata presence, and the number of alive larvae per plant. On each evaluation, 10 plants per plot were analyzed.
The insecticide efficacy (E) was calculated using Abbot [22] , as follow:
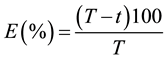
where T is the mean number of alive larvae on control treatment, and t is the mean number of alive larvae on each insecticide treatment.
2.4. Data Analysis
The data were subjected to ANOVA and the treatment means compared by Tukey test at 5% probability. To indicate the need to data transform, these were submitted to Taylor’s Power Law [23] , which indicates that percentage of attacked plants by M. vitrata should be transformed by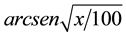 , and alive larvae by
, and alive larvae by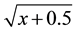 .
.
3. Results
In the first evaluation, one day after application (DAA), it was observed that 48% - 56% of the plants evaluated showed symptoms of attack by M. vitrata, considering the different treatments on the assay (Table 2). However, at four DAA was observed a significant reduction in the percentage of infested plants in relation to the control, reaching less than 20% infestation in all pesticides, and not significant difference was observed between pesticides in this evaluation date, only when compared with control (Table 2).
Seven days after treatment, it was observed that all tested pesticides remained significantly reducing the percentage of infested plants with M. vitrata compared to the control treatment (Table 2), highlighting chlorpyrifos, which provided the greatest reduction (9.60%), and methomyl, which provided the lowest reduction (19.00%). At 10 DAA was observed that chlorpyrifos showed the greatest reduction (5.40%), followed by teflubenzuron (7.20%), and chlorantraniliprole + lambda-cyhalothrin (9.40%). At 14 DAA was observed the same level of reduction of attacked plants by M. vitrata with the different pesticides used, mainly with chlorpyrifos (6.40), followed by teflubenzuron (9.20), and chlorantraniliprole + lambda-cyhalothrin (10.60), respectively (Table 2).
The number of live caterpillars encountered during the five evaluations was also significantly altered by the chemical treatment (Table 3). One day after treatment, except methomyl, all pesticides have significantly reduced the density of M. vitrata in soybean, mainly chlorpyrifos (0.00), teflubenzuron (0.40), and chlorantraniliprole + lambda-cyhalothrin (0.20) (Table 3). Four DAA, it was observed the same pattern, where pesticides chlorpyrifos (0.00), teflubenzuron (0.20), and chlorantraniliprole + lambda-cyhalothrin (0.40) showed less alive larvae (Table 3).
Seven DAA the results showed that chlorpyrifos (0.20), teflubenzuron (0.40), and chlorantraniliprole + lambda- cyhalothrin (0.60) still showed less alive larvae (Table 3). Ten DAA, the pesticide flubendiamide (0.80) joined to the group chlorpyrifos (0.40), teflubenzuron (0.40), and chlorantraniliprole + lambda-cyhalothrin (0.60), which showed the lowest number of M. vitrata larvae per plant (Table 3). 14 DAA the pesticides, chlorpyrifos (0.60), teflubenzuron (0.80), chlorantraniliprole + lambda-cyhalothrin (1.00), and flubendiamide (1.00) showed the lowest number of M. vitrata larvae per plant (Table 3).
At one DAA, chlorpyrifos (100.00) and chlorantraniliprole + lambda-cyhalothrin (93.33) showed the greatest field efficacy, and methomyl (40.00) showed the lowest field efficacy (Table 4). Four DAA, chlorpyrifos (100.00) and chlorantraniliprole + lambda-cyhalothrin (90.00) remained on the greatest field efficacy, however teflubenzuron (93.33) joined to this group (Table 4). Seven DAA, was observed the same pattern, and chlorpyrifos (95.00), chlorantraniliprole + lambda-cyhalothrin (83.33), and teflubenzuron (88.33) still showed the greatest field efficacy.
![]()
Table 2. Percentage (±SE) of attacked soybean plants by Maruca vitrata at one, four, seven, 10, and 14 days after insecticides application (DAA). Maracaju, MS, 2013.
Data within a column followed by the same letter are not significantly different (P < 0.05; Tukey test). nsNot significant. *Significant at 5% probability; **Significant at 1% probability. 1Original data. Transformed by 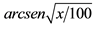 for statistical analysis.
for statistical analysis.
![]()
Table 3. Mean (±SE) of the number of alive Maruca vitrata larvae at one, four, seven, 10, and 14 days after insecticides application (DAA). Maracaju, MS, 2013.
Data within a column followed by the same letter are not significantly different (P < 0.05; Tukey test). nsNot significant. *Significant at 5% probability; **Significant at 1% probability. 1Original data. Transformed by 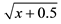 for statistical analysis.
for statistical analysis.
Ten DAA, was observed that flubendiamide (78.33) showed a significantly increase on its efficacy, reaching the more efficacy group with chlorpyrifos (90.00), chlorantraniliprole + lambda-cyhalothrin (83.33), and teflubenzuron (88.33) (Table 4). And 14 DAA, was observed the same pattern, where chlorpyrifos (86.67), chlorantraniliprole + lambda-cyhalothrin (79.33), teflubenzuron (82.67), and flubendiamide (80.33) showed the greatest field efficacy, and methomyl (54.67) showed no field efficacy (Table 4).
According to these results, it can be inferred that pesticides chlorpyrifos, teflubenzuron, and chlorantraniliprole + lambda-cyhalothrin were more efficacy on field evaluations for M. vitrata larvae control, and this effect was observed right after application (one day after treatment) in soybean. However, the pesticide flubendiamide efficacy ensured good control of this pest from 10 days after application, and final efficacy compared to pesticides chlorpyrifos, teflubenzuron, and chlorantraniliprole + lambda-cyhalothrin, fact not noted for methomyl, which showed low field efficacy on M. vitrata control.
The yield showed to be linked with the efficacy of each pesticide, where control (3,390.67) and methomyl (3,411.30) showed the lower yield, flubendiamide (3,733.33) and chlorantraniliprole + lambda-cyhalothrin (3,727.78) formed an intermediate group, and the pesticides teflubenzuron (3,881.82) and chlorpyrifos (3,989.30) had the greatest yield (Table 5).
4. Discussion
The results observed on this study indicates that the pest reached the threshold level suggested by Hoffman-Campo et al. [14] , pointed as 10% - 15% of soybean pods attacked by M. vitrata. All used pesticides reduced the pest infestation below threshold level 14 days after application, except methomyl, which showed no field efficacy against M. vitrata.
![]()
Table 4. Pesticide efficacy against Maruca vitrata at one, four, seven, 10, and 14 days after insecticides application (DAA). Maracaju, MS, 2013.
Data within a column followed by the same letter are not significantly different (P < 0.05; Tukey test). nsNot significant. *Significant at 5% probability; **Significant at 1% probability.
![]()
Table 5. Average yield (±SE) (kg・ha−1) of soybean treated with different pesticides to control M. vitrata larvae. Maracaju, MS, 2013.
Data within a column followed by the same letter are not significantly different (P < 0.05; Tukey test). nsNot significant. *Significant at 5% probability. **Significant at 1% probability.
Mandal et al. [24] observed that indoxacarb, endosulfan, lambda-cyhalothrin, triazophos, imidacloprid and thiamethoxan were effectiveness to control M. vitrata. Similar results were observed with endosulfan [25] , and with indoxacarb on blackgram [26] . Dina and Medaiyedu [27] and Jackai [28] reported that endosulfan gave effective control of the pod borer on cowpea.
On pigeonpea, some insecticides were pointed out with good efficacy for this pest, such as deltamethrin, cypermethrin, and fluvalinate [29] ; monocrotophos and endosulfan [30] ; cypermethrin and dimethoate [31] ; cypermethrin, deltamethrin, fenvalerate, and endosulfan [32] ; triazophos, endosulfan, and monocrotophos [33] ; endosulfan + miraculan (a plant growth stimulant), fenvalerate, and monocrotophos [34] ; and benomyl + monocrotophos and permethrin [35] .
Some of these insecticides are too expensive for small scale farmers and efforts are needed to avoid application of highly toxic broad spectrum insecticides. Furthermore, some insecticides are forbidden in Brazil, such as monocrotophos and endosulfan.
It was observed M. vitrata resistance to cypermethrin (17-53-fold), dimethoate (27-92-fold), and endosulfan (15-37-fold) in two locations in Nigeria (Shika and Samaru) [21] . Control failures were also reported in Kenya [36] and in Benin [37] . In Brazil, this scenario is not observed, but the present results can help on pest resistance against pesticides management.
M. vitrata has a short life cycle and high reproductive potential [38] , as a result they are frequently exposed to multiple applications of several different insecticides used for its control. Our results points out insecticides approved in Brazil and its efficacy to apply on the field to control the pod borer. We observed three different activate ingredients (chlorpyrifos, teflubenzuron, and chlorantraniliprole + lambda-cyhalothrin) with different che- mical groups (organophosphorus, benzoylurea, and anthranilamide + pyrethroid), enabling the rotation of different pesticides mode of action, and providing options for M. vitrata chemical control and resistance management.
Chemical control is an important tool in IPM systems. Using an efficacy pesticide is important to manage M. vitrata, even in cases with parasitoid releases, such as demonstrate with Trichogramma evanescens (Hymenoptera: Trichogrammatidae) inundative releases [39] .
These results point out to a pesticide recommendation to control M. vitrata on soybean, once there’s no such recommendation to control this pest. However, other studies must be done with others activate ingredients in order to provide options for growers and guarantee a more consistent recommendation to control M. vitrata.
5. Conclusions
Chlorpyrifos, teflubenzuron, and chlorantraniliprole + lambda-cyhalothrin are efficacious on M. vitrata control of field spray right after the application, and flubendiamide will be efficacious 10 days after application.
Methomyl is not efficacious on M. vitrata control of field spray.