Protein Quality of Amaranth Grains Cultivated in Ethiopia as Affected by Popping and Fermentation ()
1. Introduction
The genus Amaranthus belongs to the family Amaranthaceae. It has a huge biodiversity and several of them are cultivated as leafy vegetables, grains, and ornamental plants while others are weeds [1] . Among all the species, Amaranthus caudatus, Amaranthus hypochondriacus and Amaranthus cruentus are mainly cultivated for their seeds [2] [3] .
Amaranth was neglected from the food table for many decades after the arrival of the Spanish conquistadors in Latin America as it was used in ceremonial dishes associated with human sacrifice by the Aztecs [1] . It is known for its high tolerance to arid conditions and poor soils, its resistance to drought, heat, and pests, and its ability to adapt to environments where conventional cereal crops do not grow well. Amaranth can thus contribute to food security, especially in resource poor setting [1] [4] . Despite the very wide distribution of amaranth in Ethiopia, it is cultivated as intercropped with sorghum and maize mainly by Me’enit people who live in the Southern Nations, Nationalities and Peoples Region (SNNPR). The most frequent mode of consumption of amaranth grains by these people is after popping and milling, and by mixing the flour with other cereal flours such as teff, sorghum, barley and wheat to prepare bread, injera and porridge.
Concerning the nutritional quality, protein content of amaranth grains is higher than that of most common cereals [5] [6] . In addition, amaranth grains contain useful amino acid profile [6] - [8] . Despite the high protein content and interesting amino acid profile of amaranth grains, the overall protein quality is dependent on the digestibility. In this regard, popping, the most commonly used processing method for amaranth, and fermentation, which is widely used in Africa to prepare cereal based foods like injera, can influence protein digestibility [9] - [12] . This is due to the fact that these techniques are associated with the decrease in the content of exogen- ous factors such as tannin, phytate and trypsin inhibitors which are responsible for reducing protein digestibility.
Other processing techniques such as boiling, microwave cooking, and autoclaving also affect protein digesti- bility. According to El-Adawy et al. (2002) [10] , boiling, microwave cooking and autoclaving improved the digestibility of chickpea protein. On the other hand, Nunes et al. (2004) [13] reported that cooking decreased the digestibility of sorghum and maize protein. Similar controversies surround the effect of popping on the digesti- bility of amaranth protein. According to a study conducted by Gamel et al. (2004) [6] , popping improved the IVPD whereas Pedersen et al. (1987) [5] and Písaříková et al. (2005) [8] reported a decrease in IVPD during popping of amaranth. On the other hand, fermentation has been found to improve in vitro protein digestibility of cereals due to the degradation of protein binding molecules [9] [11] [12] .
In developing countries like Ethiopia, complementary foods for young children are mainly prepared from mixes of cereals and legumes. It is known that among essential amino acids, legumes are rich sources of lysine but lack methionine and cysteine, while cereals are rich in these two amino acids but are poor sources of lysine. Mixing legumes and cereals during complementary food formulation is a common strategy to meet essential amino acid requirements. However, a too high proportion of legumes sometimes compromise the acceptability of the product and, in addition, legumes contain high levels of antinutritional factors. It is thus advisable to reduce the proportion of legumes in complementary food formulation.
The aim of the present study was, therefore, to evaluate the protein quality of three different varieties of ama- ranth grains cultivated in Ethiopia in the raw state and after popping or fermentation by analyzing the contents of free and total amino acids and in vitro protein digestibility of amaranth grain porridge to assess its potential application as an ingredient for complementary food formulation.
2. Materials and Methods
2.1. Materials
Three different varieties of amaranth grains, white, red and brown in color, were purchased from six farmers in Chat Kebelle, Bench Majji Zone, in the Southern Nations, Nationalities and Peoples region, Ethiopia. Composite samples of each variety prepared from the grains of the six origins were sorted, cleaned and washed to remove immature seeds, sand and soil. The washed seeds were sun dried, milled and sieved in a 0.425 mm sieve. The flour was stored at 4˚C until further analysis.
2.2. Solvents, Chemicals and Reagents
Citrate buffer pH 2.2 and ninhydrin reagent were purchased from Biochrom (France). Sodium hydroxide (BioXtra, ≥98% acidimetric), pellets (anhydrous), L-Norleucine (N8513 suitable for amino acid analysis), aminobutyric acid (A2129; ≥99%), methanesulfonic acid (M4141; 4 M with 0.2% (w/v) tryptamine) were purchased from Sigma-Aldrich (Saint-Louis, Missouri, USA). Amino acid standards (AA-S18; analytical standard), hydrochloric acid 0.1 N were purchased from Fluka (Fluka Chemicals, Buchs, Switzerland). All reagents and chemicals used were of analytical grade.
2.3. Processing Methods
2.3.1. Popping
Sun dried seeds were placed in a hot clay pan for 10 - 15 seconds until they popped. The popped grains were milled to pass through a 0.425 mm sieve and stored in polyethylene bags at 4˚C.
2.3.2. Fermentation
Natural fermentation was carried out according to the method described in [14] with modifications. Briefly, 250 g of flour prepared from raw amaranth grains was mixed with 500 ml distilled water and then left to ferment spontaneously for 48 h at room temperature. The sample was mixed, transferred into aluminum cups and dried in a hot oven (Heraeus UT 5042, Germany) at 50˚C for 20 h. The dried sample was then ground to pass through a 0.425 mm sieve and stored in polyethylene bags at 4˚C.
2.3.3. Preparation of Gruels
Amaranth gruels were prepared using the method described in [15] . Briefly, the flour was mixed with cold deionized water into slurry and cooked with continuous stirring over a hot plate for 5 min after the mixture starts to boil. The gruel was allowed to cool and sample was taken for protein digestibility experiment.
2.4. Chemical Analysis
2.4.1. Protein and Dry Matter Measurement
The nitrogen content of all Samples was determined using Kjeldhal method and a conversion factor of 5.85 was used [16] . The dry matter content was determined by oven drying at 105˚C to a constant weight.
2.4.2. Free and Total Amino Acid Analysis
1) Extraction procedure
a) Free amino acids
Free amino acids were analyzed following the method used by Moore et al. (1958) [17] with modifications. Briefly, 150 mg of sample was weighed and placed in a sealable test tube. To this, 50 µl of internal standard Norleucine (25 µM) and 4.95 ml of citrate buffer (pH 2.2) were added. The solution was mixed for 1 h on a rotational shaker. All extractions were performed in triplicate.
b) Total amino acids
Samples (10 - 20 mg) of dried flour were weighed in a Schlenk tube and 50 µl of 25 µM Norleucine and 450 µL of 4 M methanesulfonic acid were added. The tube was flushed with nitrogen, closed and heated at 150˚C for 2 h. After cooling, 450 µL of 4 M NaOH was added to the hydrolysate, which was diluted to 5 ml with a loading buffer (citrate buffer at pH 2.2). All extractions were performed in triplicate.
2) Amino acid analysis
Sample extracts for free and total amino acid analysis were filtered using a 0.45 µm membrane filter and injected into the amino acid analyzer (Biochrom 30+, Biochrom, France), using a lithium cation exchange resin column and ninhydrin as detection compound. All total and free amino acid contents were determined except total tryptophan, which is the most fragile amino acid and is destroyed by the extraction procedure. Amino acid standards were also run in a similar way as the samples.
2.4.3. Determination of in Vitro Protein Digestibility
In vitro protein digestibility was determined according to the method of Akeson and Stahmanna (1964) [18] with modifications. To 10 g of amaranth porridge (with approximately 10% assumed DM content), 15 ml of 0.16 M HCl containing 1.5 mg pepsin (Sigma, P-7000, 14,900 u/mL) was added and incubated at 37˚C for 2 h in a shaking water bath. The resulting suspension was neutralized with 7.5 ml of 0.32 M NaOH and treated with 4 mg of pancreatin (Sigma, P-7545, 8*USP specifications) in 7.5 ml of 0.32 M phosphate buffer (pH 8.0). The mixture was incubated for an additional 2 h at 37˚C. Enzyme blank was prepared by incubation under the same conditions except that the sample was omitted. After incubation, the sample was treated with 10 ml of 10% trichloroacetic acid (Sigma) to remove undigested protein and larger peptides and centrifuged at 5000 g for 20 min at room temperature. The solution was filtered using Whatman No. 1 filter paper, and the protein in the supernatant was then determined using Kjeldahl method.
2.4.4. Amino Acid Score and Protein Digestibility Corrected Amino Acid Score
The amino acid score (AAS) is the ratio of the amino acid content in the protein of a food/diet to the content of the same amino acid in the requirement pattern. The score determines the effectiveness with which absorbed dietary nitrogen can meet the indispensable amino acid requirements at the safe level of protein intake. This is thus a measure of the actual amounts of individual amino acids in a food with respect to the need for this amino acid. And the quality of the protein will finally depend on the indispensable amino acid for which the AAS is the lowest. However, AAS does not account for the digestibility of the protein. Therefore, another scale called the protein digestibility corrected amino acid score (PDCAAS) was adopted by WHO/FAO (2007) [19] to better explain digestibility in relation to the needs of humans and the scoring of foods (Equation (1)):
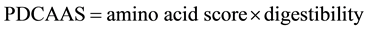 (1)
(1)
2.5. Calculation of the Percentage Contribution of Amaranth to Estimated Amino Acid Requirements
From the amino acid data, intakes of indispensable amino acids were calculated for comparison with the WHO estimated needs [19] , assuming breast-fed infants aged 6 - 23 months consume the recommended daily ration size of 50 g/d dry weight complementary food [20] . The amount of cereal used in complementary food formulation was assumed to be 64% [21] and, assuming that this amount of cereal is replaced by amaranth, its percent contribution to the daily indispensable amino acid requirements was calculated.
2.6. Data Analysis
All measurements were done in triplicate and statistical analyses were performed using the software Statgra- phics plus 5.1 (Statpoint, Warrenton, USA). Two-way ANOVA was applied to the experimental data and means were separated using Fischer’s least significant difference tests with a probability p < 0.05.
3. Results and Discussion
3.1. Protein, Total and Free Amino Acid Content in Amaranth Grains
Table 1 indicates the crude protein, and total and free amino acid contents in raw amaranth grains. The protein contents of raw white, red and brown amaranth were 13.9, 15.2 and 15.5 g/100g DM, respectively. These re- sults are in agreement with the results reported by Pedersen et al., (1987) [5] . Kaur et al. (2010) [3] also reported that 14.8% - 16.8% protein has been obtained from 11 lines of Amaranthus caudatus species grown in India.
The amount of almost all essential amino acids in the raw samples was above the new FAO/WHO standard pattern for all age groups [19] implying that there is no limiting amino acid in amaranth grains considered in this study. Tryptophan, one of the indispensable amino acids, was not analyzed in this study but Gamel et al. (2004) [6] reported that their amaranth samples also contained appreciable amounts of tryptophan.
The lysine content of raw amaranth ranged from 65 to 74 mg∙g−1, protein which is close to the lysine contents in legumes (70 - 75 mg∙g−1 protein) including chickpeas, cowpeas and lentils but twice higher than that found in many other cereals, such as barley, wheat, rice, rye and maize: 22 - 37 mg∙g−1 protein. Methionine and cysteine contents were ranged from 24 to 29 and 43 to 51 mg∙g−1 protein, respectively, which were higher than that found in the legumes and cereals mentioned above [22] [23] . The concentrations of essential amino acid in amaranth grains cultivated in Ethiopia (Table 1) were in good agreement with those reported by Chavez-Jauregui et al. (2000) [24] and slightly higher than those reported by Gamel et al. (2004) [6] in Amaranthus caudatus.
The percentage of indispensable amino acids, excluding tryptophan, tryptophan, was highest in raw white
![]()
Table 1. Total and free amino acid profile (mg∙g−1 protein) of three varieties of raw amaranth grains†.
†Values are means of results obtained in triplicate ± SD expressed in mg∙g−1 protein and means followed by different letters in the same row are significantly different at p < 0.05. §AAA: aromatic amino acids (phenylalanine and tyrosine). ¥SAA: sulfur amino acids (cysteine and methionine). ND: not determined. *Indispensable amino acids [19] . ¶Standard FAO/WHO reference pattern for indispensable amino acids for children aged 1 - 2 years [19] .
amaranth (49%) followed by 43% in both raw red and brown amaranth. These values are higher than the reference protein pattern for 1 - 2 years old children (31%). The amaranth amino acid profile thus provides a good balance of total indispensable amino acids, and some of the limiting amino acids, especially lysine in cereals and methionine and cysteine in legumes, could be complemented by amaranth.
The free amino acid content of the three varieties of raw amaranth is listed in Table 1. The main essential free amino acid was histidine followed by lysine and tryptophan with 1.2 - 1.5, 0.9 - 1.2 and 0.7 - 1.4 mg∙g−1 protein, respectively. A study by Nimbalkar et al. (2012) [25] on Amaranthus hypocondriacus grain reported that the top three essential free amino acids were threonine, phenylalanine, and methionine and that the amount of threonine was twice that of the other free amino acids. However, in the present study, no free threonine was detected in any of the three varieties of amaranth grains, and glutamic acid and proline were the main non-essential free amino acids with values in the range of 1.8 to 4.6 and 1.5 to 3.7 mg∙g−1 protein, respectively.
3.2. Effect of Popping and Fermentation on Total Amino Acid Content
Popping decreased the amount of six amino acids including three indispensable amino acids: lysine, methionine, and cysteine. The decrease was highest for lysine and cysteine and reached 36% and 37% reduction, respec- tively (Table 2). In a study conducted in two different species of amaranth (Amaranthus caudatus and Amaran- thus cruentus) by Gamel et al. (2004) [6] , the highest loss of amino acid was recorded for tyrosine followed by phenylalanine and methionine with 35%, 24% and 18% loss for Amaranthus caudatus and 32%, 20% and 19% for Amaranthus cruentus, respectively. Another study by Tovar et al. (1989) [26] reported highest loss for lysine (56%) followed by arginine (29%). Pisarikova et al. (2005) [8] also reported that the decrease in the concentra- tion of indispensable amino acids due to heat treatment was highest for histidine followed by lysine and leucine. Despite the improvement in sensory attributes during popping, the associated loss of amino acids decreased the overall quality of amaranth proteins.
In the present study, fermentation also led to a significant decrease in the contents of the same six amino acids as that of popping (Table 2). However, after fermentation, the decrease in essential amino acids, lysine and cysteine with percent reduction of 20 and 16%, respectively, were significantly lower than after popping (p < 0.05) and methionine was subject to a higher (20%) percent reduction although not statistically significant (p < 0.05). The reduction could be ascribed to metabolism of amino acids by microorganisms into ammonia and volatile compounds that are responsible for the flavor of the fermented product [12] .
3.3. Effect of Popping and Fermentation on Free Amino Acid Content
Popping and fermentation led to highly significant changes in free amino acid contents (p < 0.01) (Table 3).
![]()
Table 2. Effect of popping and fermentation on total amino acid content (mg∙g−1 protein) of amaranth grains†.
†Values are means of results obtained in triplicate ± SD for the three amaranth varieties. *The effect of processing is significant (p < 0.05) and means followed by different letters in the same row are significantly different at p < 0.05.
![]()
Table 3. Effect of popping and fermentation on free amino acid content (mg∙g−1 protein) of amaranth grains†.
†Values are means of results obtained in triplicate ± SD expressed for the three amaranth varieties. Means followed by different letters in the same row are significantly different at p < 0.01.
Popping led to a significant decrease in almost all free amino acid contents (Table 3). Free aromatic amino acids were strongly affected, phenylalanine and tyrosine completely vanished and 90% of tryptophan vanished during popping. Non-enzymatic browning reaction is the most probable explanation for the decrease in the level of free amino acids during heat treatment [27] .
Conversely, fermentation increased the amount of almost all free amino acids, except tyrosine, glutamic acid and proline, which remained unchanged, and arginine, which strongly decreased (Table 3). Lysine and phenylalanine increased to a greater extent than the other free amino acids. Increased microbial enzyme activity coupled with protein hydrolysis is the likely explanation for the increase in the amount of free amino acids during fermentation [12] [28] . Hamad and Fields (1979) [29] also showed that there was a significant increase in free lysine content during fermentation in other type of cereals.
3.4. Effect of Popping and Fermentation on in Vitro Protein Digestibility (IVPD) and Protein Digestibility Corrected Amino Acid Score (PDCAAS)
In vitro protein digestibility (IVPD) is shown in Figure 1. When the digestibility of porridge made from raw amaranth samples was compared, it was highest for white (82.4%) followed by red (77.6%) and brown (71.2%) varieties. The lower IVPD in the colored varieties might be related to the presence of higher amount of dietary
![]()
Figure 1. Effect of processing on in vitro protein digestibility (%) in amaranth. Means with different letters are significantly different at p < 0.05 for the same variety.
fiber and polyphenols (data not shown), which could bind the protein and reduce its susceptibility to enzymatic attack [5] .
Both popping and fermentation significantly affected, (p < 0.05), the IVPD (Figure 1). Popping decreased protein digestibility by 17.14%, 8.3% and 9.8% for white, red and brown colored amaranth, respectively. This is because amino acids undergo several chemical reactions during severe heat treatment, including Maillard reaction between amino acids and reducing sugars, which reduces the availability of amino acids [27] . Therefore, during dry heat processing (popping) where the temperature often exceeds 150˚C, this reaction is likely to contribute to the decrease in protein digestibility. Unlike popping, fermentation improved IVPD by 4.8%, 5.9% and 7.5% for white, red and brown colored amaranth, respectively. This could be due to degradation of phytic acid (data not shown), which is a potent inhibitor of proteolytic enzymes. Besides, hydrolysis of tannins, which potentially complex proteins, may also increase the accessibility of protein molecules for enzymatic attack and hence increase IVPD [30] .
In addition, hydrolysis of proteins by microorganisms during fermentation, evidenced by the increase in free amino acids, could increase IVPD. Studies by El Hag et al. (2002) [11] , Pranoto et al. (2013) [12] and Hassan and El Tinay (1995) [9] on finger millet and sorghum, indeed showed that tannin hydrolysis occurs under the action of microorganisms during fermentation.
For all the indispensable amino acids, the PDCAAS, which takes both the amino acid score and protein diges- tibility into account, was highest for the white followed by the red and brown variety (Table 4). In all the three varieties, the popped samples had the lowest PDCAAS values due to the loss of heat labile amino acids during popping. Although there was a significant improvement (p < 0.05) in protein digestibility during fermentation (Figure 1), the PDCAAS of almost all essential amino acids slightly increased or remained unchanged after fermentation except lysine in the brown variety, which decreased considerably. In the white and red varieties, leucine in raw samples, leucine and lysine in popped samples and leucine in fermented samples were decreased, while in the brown variety, leucine in raw samples and lysine in popped and fermented samples were decreased.
3.5. Percentage Contribution of Amaranth as an Ingredient in Complementary Foods to the Daily Amino Acid Requirement of 6 - 23 Months old Children
Table 5 shows the percent contribution of amaranth to the daily indispensable amino acid requirements of 6 - 23 months old children. All the three varieties of amaranth contributed more than 50% of the daily indispensable amino acid requirements based on the consumption of 50 g of complementary foods per day of which amaranth accounts for 32 g. Compared to the cereals commonly used in complementary food formulation such as wheat and maize, the contribution of amaranth is appreciably higher for all indispensable amino acids except leucine
![]()
Table 4. Percent protein digestibility corrected amino acid score (PDCAAS) for three varieties of raw and processed amaranth grains*.
*The scoring pattern for indispensable amino acids was considered for children aged 1 - 2 years [19] . ¥SAA: sulfur amino acids (cysteine and methionine). §AAA: aromatic amino acids (phenylalanine and tyrosine).
![]()
Table 5. Percentage contribution of raw amaranth to the daily indispensable amino acid requirements for children aged 6 - 23 months, compared to that of more commonly used cereals (maize or wheat)*.
*Assuming daily consumption of 50 g of complementary food on a dry weight basis formulated with 64% of cereals or amaranth. †Shewry et al. (2007) [23] . $The lower and upper values refer to the requirements for 6 and 23 months old children, respectively [19] . ¥The lower and upper values refer to the contribution of unprocessed cereals to the daily amino acid needs for 23 and 6 months old children, respectively.
(Table 5). Moreover, the daily needs of sulfur-containing amino acids were fully met by consuming the suggested amount of amaranth. However, if only the digestible fraction of protein is taken into consideration, the contribution to amino acid requirements would decrease by about 18% - 29%.
4. Conclusion
All three varieties of amaranth investigated in this study can be considered as a potential source of protein. Due to its high essential amino acid contents, amaranth could potentially substitute other more common cereals used in complementary food formulation for young children and thus reduce the proportion of legumes. Although popping improved the sensorial attributes of amaranth porridge, it reduced protein quality through the loss of heat labile amino acids. On the other hand, fermentation was better than popping in maintaining the amino acid profile with its added advantage of improving the protein digestibility although it resulted in a marked decrease in lysine content.
Acknowledgements
The authors are grateful to Addis Ababa University, SIDA SAREC Swedish government, and Institut de Recherche pour le Development (IRD), Montpellier, France for supporting the study.
NOTES
*Corresponding author.