1. Introduction
Previous studies have demonstrated that several fruit phytochemicals exert antioxidant and anti-inflammatory properties [1] [2] . Beyond antioxidant properties, some phytochemicals display hypoglycaemic activities [3] [4] . Recent studies have also shown that some fruit phenolics inhibit the formation of advanced glycation end products (AGEs) [5] -[10] , whose formation and accumulation exert a pathophysiological role in patients with diabetes, renal and neurodegenerative disease as well as some types of cancer [11] [12] . AGEs, produced through non-enzymatic reactions between reducing sugars and proteins, nucleic acids or lipids, induce oxidative stress and molecular cross linkages that cause cellular and tissue damage by impairing protein function and clearance [13] [14] . The biological activities exerted by fruit phytochemicals are believed to contribute to the overall health-protective effect of fruits and vegetables because they may mitigate the consequences of oxidative stress in disease development and aging process [15] [16] .
Among fruits, sweet and sour cherry (Prunus spp.) phytochemicals have been previously characterized. The phenolic composition in sour and sweet cherries includes flavonoids such as anthocyanins, flavan-3-ols, flavonols and phenolics acids whose biological properties have been demonstrated in different experimental models and reviewed [17] [19] . Antioxidant properties of cherry phytonutrients have been reported both in vitro [17] - [22] and in vivo studies [23] -[25] . Several studies have demonstrated that the synthesis of antioxidant phytochemicals is modulated by genetic and environmental factors such as fruit variety, maturity stage and processing [17] [26] [27] . Studies of ripening are of special interest because they allow the identification of the optimum point of maturity for harvesting and enable delivery of fruit to consumers in its best condition in terms of nutritional and functional properties. Little is known about the effect of ripening on phytonutrients contained in the local variety of sour cherry (Prunus cerasus L.) morellos, var. austere L., known in Marche region, Italy, as “visciola”.
Aim of this study was to investigate the effect of the maturity stage on phenol content and nutraceutical properties of this variety of sour cherry. Therefore, we evaluated the anti-glycation and antioxidant properties of fruit extracts using methylglyoxal-glycated albumin and copper catalyzed oxidation of human low density lipoproteins (LDL), as experimental models.
2. Materials and Methods
2.1. Chemicals
All reagents, including fluorescein, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,20- azobis(2-methylpropionamidine)dihydrochloride (AAPH), copper-sulfate, methylglyoxal (MGO), serum bovine albumin (BSA), Folin-Ciocalteau reagent, gallic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH) and solvents were purchased from Sigma-Aldrich Chemical Co (Milan, Italy).
2.2. Fruit Soluble Solids, Titratable Acidity
Sour cherries (Prunus cerasus L.) local Morellos type “visciola” of two native different clones (A and B) were from an experimental farm in Castelbellino (AN, Italy) and were harvested in 2009 at two different stages of ripeness: partially ripen (PR) and fully ripen (FN). Acidity was titrated using 10% NaOH water solution and expressed as tartaric acid (g/L) while soluble solid content of the juice (˚brix) was determined using a hand refractometer (Hand refractometer, Atago LTD, Tokyo, JP).
“Visciola” samples (at least 30 fruits) were pitted, packed and stored at −20˚C until analyzed.
2.3. Determination of Total Phenol Content
“Visciola” (approximately 20 mg) were homogenized using an Ultra-Turrax T25 for 5 min at 30 Hz in 2 mL ice-cold 50% acetone. To avoid degradation of pigments and phenolic compounds, the homogenization was carried out in ice and darkness. Samples were centrifuged (4500 g for 30 min at 4˚C) and filtered through Whatman n. 4 filter paper.
Total phenol content of the extracts was determined using the Folin-Ciocalteu reagent [28] . Briefly, appropriately diluted extracts (0.050 mL) and 1.5 mL of water were added followed by 0.125 mL of Folin-Ciocalteau reagent and mixed. After 1 min, 0.375 mL of 20% Na2CO3 and 0.450 mL of water were added to reach a final volume of 2.5 mL. After mixing, samples were left for 2 hrs at room temperature in the dark. The absorbance was then read at 760 nm against blank. The results were expressed as mg gallic acid equivalents (GAE) in 100 g of fruit [28] .
2.4. Determination of Total Antioxidant Capacity
Total antioxidant capacity of the fruit extracts was evaluated using oxygen radical absorbance capacity method (ORAC) and by scavenging of the radical 2,2-diphenyl-1-picrylhydrazyl (DPPH).
ORAC assay was carried out on a plate reader [29] . Briefly, AAPH was used as peroxyl radical generator, trolox as standard, and fluorescein as fluorescent probe. Fluorescence filters were used for an excitation wavelength of 485 nm and an emission wavelength of 530 nm. 20 µL of diluted sample, blank, or trolox calibration solutions were mixed with 150 µL of 0.08 µM fluorescein and incubated for 15 min at 37˚C before injection of 25 µL of AAPH solution (150 mM). The fluorescence was measured every 2 min for 4 h. All samples were analyzed in triplicate. The final ORAC values were calculated using the net area under the decay curves and were expressed as millimoles of Trolox equivalents (TE)/100g of fruit.
Radical scavenging activity of samples was evaluated by monitoring the decrease of absorbance at 517 nm of methanolic solution of the radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) (stock solution 200 mM) incubated for 5 minutes in the absence (Ab) or in the presence (Ac) of samples. Radical scavenging activity was calculated by the following formula: % inhibition = [(Ab − Ac)/Ab] × 100. Results were expressed as micromoles of Trolox equivalents (TE)/100g of fruit [30] .
2.5. LDL Isolation and Oxidation
LDL particles were isolated from pooled fresh human blood obtained from volunteers after overnight fasting. Blood was collected in heparin-containing vacutainers and plasma was obtained by centrifugation at 3000 rpm for 15 minutes. LDL (density between 1.025 and 1.063 g/ml) were isolated by single vertical spin gradient ultracentrifugation on a Sorvall RC-150GX ultracentrifuge following the procedure described by Chung et al. [31] . After dialysis at 4˚C for 24 h against 5 mM PBS pH 7.4, protein concentration was determined by the method of Bradford [32] . Antioxidant activities were assessed by direct monitoring of the retardation of conjugated diene formation at 234 nm during copper-induced oxidation of human LDL [33] . Conjugated dienes were evaluated in LDL incubated in the presence and absence of fruit extracts in a 96-well microplate reader. Briefly 0.01 mL of extract were added to 0.02 mL of LDL (1 mg protein/mL). The volume of each well was adjusted with deionized water to reach the final volume of 0.2 mL. The oxidation reaction was initiated by adding 0.01 ml of 0.1 mM Cu2+ (final concentration 5 µM) to each well and conjugated dienes were monitored at 234 nm for 4 h at 37˚C.
2.6. In Vitro Glycation of BSA
The non-enzymatic glycation of BSA was induced in vitro using methylglyoxal (MGO). Briefly BSA (1 mg/mL) was incubated with MGO to a final concentration of 5 mM for 72 hours at 37˚C in presence of sodium azide (0.02%) [34] . The incubation was carried out in the absence (MGO-BSA) or in the presence of fruit extract (MGO-BSA-SAMPLE). Different concentrations of the extracts were used (0.1 - 10 mg/mL).
The reaction of non-enzymatic glycation of samples was investigated by evaluating the increase in intrinsic fluorescence associated to the formation of advanced glycation fluorescent products. Samples were analyzed with an excitation/emission wavelength pair 365 nm/440 nm.
Results were reported as percent inhibition of MGO-induced glycation of BSA where percent inhibition = [Intensity (MGO-BSA-SAMPLE) − Intensity (BSA-SAMPLE)]/[(Intensity (MGO-BSA) − Intensity (BSA)] × 100.
The amount of sample (mg) required to inhibit the reaction rate by 50%, I50 was interpolated.
2.7. Tryptophan Fluorescence
Tryptophan emission florescence spectra of BSA incubated in different experimental conditions was evaluated using 295 nm as excitation wavelength [35] .
2.8. Statistical Analysis
Mean value and standard deviation (SD) were calculated. ANOVA analysis and Duncan’s multiple range test for mean separation were performed and a p < 0.05 was considered statistically significant.
3. Results
The fresh weight of the fruit pulp was very similar in the two “visciola” selections (clone A and clone B) with a slight increase, but not significant, in the final stage of fruit ripening. The average fresh weight for “visciola” was around 2 g. Soluble solids content significantly increased with maturity, while the titratable acidity was not significantly modified (Figure 1(a) and Figure 1(b)).
No significant differences were observed in the total content of phenol compounds among the two selections of “visciola” (clone A and clone B) (data not shown). As summarized in Table 1, the total phenol content was significantly higher in FR fruits (p < 0.001). Table 1 summarizes total antioxidant capacity evaluated by ORAC assay and DPPH assay. ORAC values were higher in fruits ripened at a more advanced maturity stage (FR compared with PR) (p < 0.001). These results were confirmed using the DPPH assay (p < 0.001). A significant correlation was observed between the ORAC values and DPPH scavenging activity (r = 0.95, p < 0.001).
3.1. Effect of Sour Cherry Extracts on MGO-Induced Glycation of BSA
As shown in Figure 2, a concentration-dependent inhibitory effect on AGEs formation (from 0.1 mg/mL to 10 mg/mL) was exerted by “visciola” extracts. At higher concentration the inhibitory effect exerted by FR fruit reached 80%. The inhibitory activity was lower in PR “visciola “than in FR fruits (IC50 = 8.8 ± 0.3 mg/mL in PR fruits and 5.8 ± 0.2 mg/mL in FR fruits, p < 0.001).
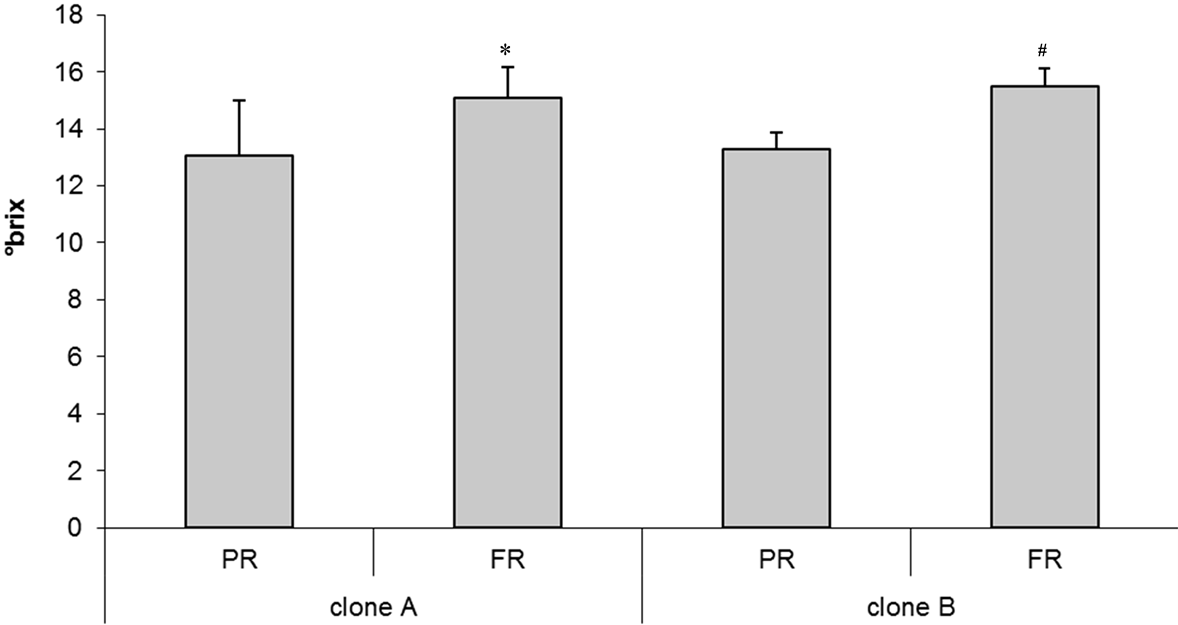 (a)
(a) (b)
(b)
Figure 1. (a) Soluble solids content (˚brix) and (b) acidity expressed as tartaric acid (g/L) in “visciola” (clone A and clone B) at different maturity stage (PR, partially ripen and FR, fully ripen). (*p < 0.05 vs FR clone A; #p < 0.05 vs FR clone B).

Table 1. Total phenol content and total antioxidant capacity evaluated by ORAC assay and DPPH assay in “visciola” at two different maturity stage (partially ripen and fully ripen) in fresh and dried fruits.
*p < 0.05 vs fruit partially ripened.
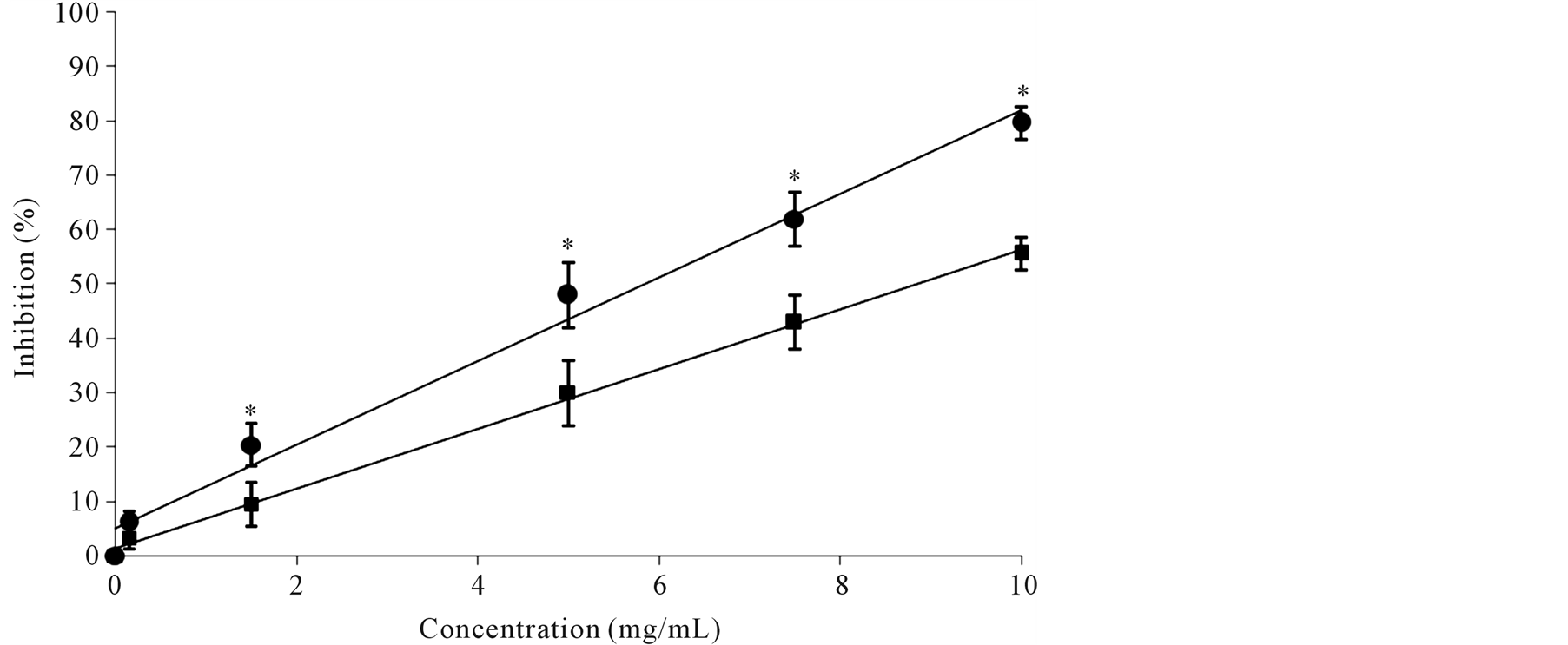
Figure 2. Protective effect of “visciola” extracts against non-enzymatic glycation of BSA induced by methylglyoxal (MGO-BSA). Dose-response curves for AGEs formation in MGO-BSA incubated in the presence of fruit extracts at different maturity stage: fully ripen, FR ( ) or partially ripen, PR (
) or partially ripen, PR ( ). *p < 0.05 vs FR fruit.
). *p < 0.05 vs FR fruit.
Tryptophan fluorescence intensity of BSA (875 ± 45 A.U.) was quenched by more than 70% after glycation with methylglyoxal (MGO-BSA) (232 ± 54 A.U.) (p < 0.001). No significant modification was shown in BSA glycated in the presence of fruit extract at all the concentrations employed with respect to MGO-BSA (data not shown).
3.2. Effect of Sour Cherry Extracts on LDL Oxidation
Lag-time of conjugated diene formation during Cu2+-oxidation of LDL is a widely used index to investigate the kinetic of oxidation of LDL and to evaluate the antioxidant capacity of fruit extracts [21] [36] . Lag time in Cu2+- oxidized LDL was 69 ± 12 minutes (Figure 3). In LDL oxidized in the presence of fruit extracts lag time was longer suggesting a protective effect against Cu2+-triggered lipid peroxidation of LDL. Extract of PR fruit exerted a lower protective effect compared with FR fruits (Figure 3).
4. Discussion
Previous studies have shown that the content of nutrients in fruits is affected by the degree of maturity at harvest, genetic differences (cultivar) and processing, however, their concentration varies from plant to plant or even in different organs of the same plant at different ripening stages [17] [26] [27] .
We studied phenol content and nutraceutical properties of the local germoplasm of sour cherry “visciola” from Marche (Italy). During the maturation the soluble solids in “visciola” increased without any decrease of titratable acidity. Higher levels of phenols have been measured in fully ripened “visciola” with respect to partially ripened “visciola”. These data demonstrate that ripeness stage has a high influence on phenol levels of “visciola”, in good agreement with data obtained in other fruits [19] [37] [38] . In fact maturation of fruit or other plant tissues
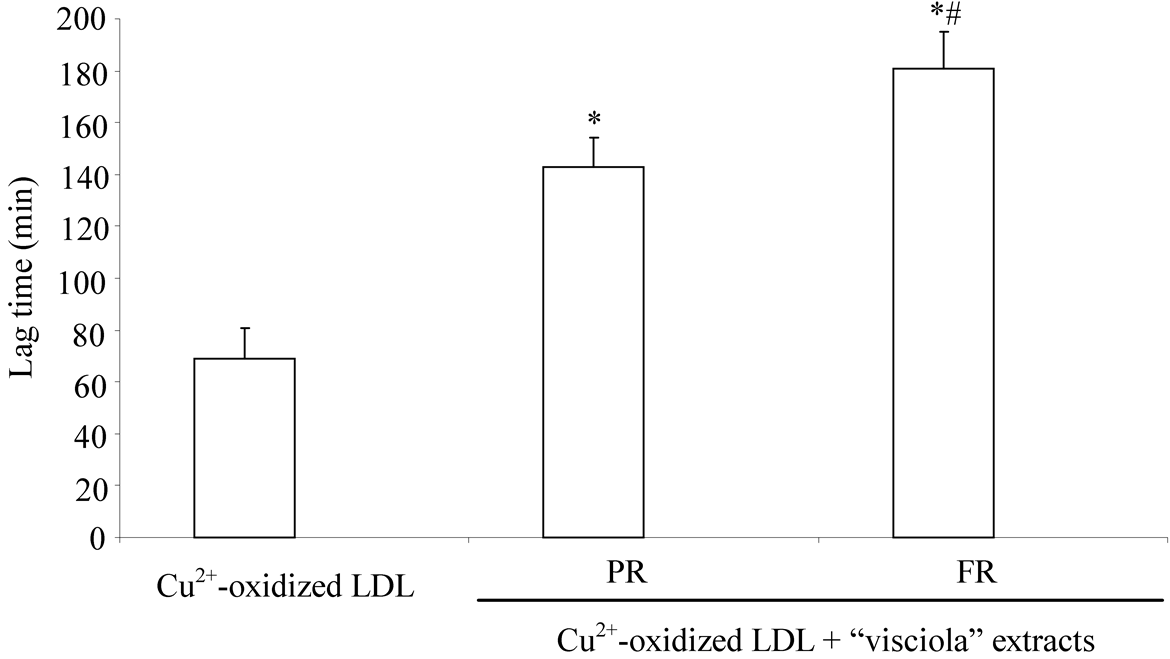
Figure 3. Protective effect of “visciola” extracts against lipid peroxidation of LDL induced by copper ions. LDL oxidized in the absence (Cu2+-oxidized LDL) or in the presence of fruit extracts at different maturity stage: PR, partially ripen and FR, fully ripen. (*p < 0.05 vs Cu2+-oxidized LDL; #p < 0.05 vs Cu2+-oxidized LDL incubated in the presence of PR fruit).
involves a series of complex reactions, which leads to changes in the phytochemistry of the plants. In particular, Serrano et al. have demonstrated that the enhancement in total phenolics observed in different sweet cherry cultivars at higher maturity stages was positively correlated with the increase in total anthocyanins [38] .
Also the values of antioxidant potential (ORAC assay and DPPH assay) were higher in fully ripened fruits compared with partially ripened fruits. In fully ripened fresh fruits ORAC value was about 2.5 mmol Trolox equivalents (TE)/100g, in agreement with previous studies in different sour cherries such as Amarena Mattarello, Visciola Ninno, Visciola Sannicandro, Montmorency and Balaton cherries [20] [22] [39] .
To further investigate the effect of maturity stage on biological properties of “visciola”, we studied the effect of fruit extracts against the formation of advanced glycation end products in BSA glycated in vitro by MGO and against lipid peroxidation of human LDL induced in vitro by copper ions.
We demonstrated that “visciola” extracts exerted a protective effect against MGO-glycation of BSA. The effect realized at a higher extent in extracts obtained from fully ripened fruits. Glycation is a major source of reactive oxygen species (ROS) and reactive carbonyl species (RCS) that are generated by oxidative (glycoxidative) and non-oxidative pathways [14] [40] . Reactive dicarbonyl species, such as MGO, have received extensive attention because these compounds are highly reactive and can form AGEs with proteins, phospholipids and DNA. Structural and functional alterations have been observed in MGO-glycated protein [13] [41] [42] . Advanced glycation end-products are well-known contributors to the pathophysiology of aging and diabetic chronic complications [11] [12] [43] . Therefore, natural products containing molecules able to inhibit AGEs formation are obtaining an increasing attention. Inhibitors of glycation can act in multiple steps [10] .
Previous studies have demonstrated that tryptophan fluorescence can be quenched by changes in the protein structure induced by MGO [35] . In agreement with other authors, we have shown that incubation of BSA with MGO produces dramatic changes in tryptophan fluorescence [35] . Fruit extracts displayed any significant effect on these changes which rules out any significant participation as inhibitors in the first phase of the glycation cascade. Therefore, we suggest that the inhibitory effect is due mainly to an inhibition of the second phase of the glycation reactions, namely the free-radical mediated conversion of the Amadori products to AGEs. These data suggest that fruit extracts exert these effects mainly through their antioxidant and free radical quenching capacity as reported by other authors [35] . Moreover, we observed “visciola” extracts are able to inhibit Cu2+-induced lipid peroxidation of LDL. In our experimental conditions both anti-glycation effect and antioxidant property were higher in extracts obtained by fully ripened fruits.
Fruit phytochemicals able to exert antioxidant effects have been previously studied. Not all phenol compounds in sour cherries are equally effective as antioxidants [20] [21] [36] . In LDL oxidized by copper ions, Heinonen et al. have shown that berry extracts inhibited hexanal formation in the order: blackberries > red raspberries > sweet cherries > blueberries > strawberries and that the antioxidant activity was associated directly with anthocyanins and indirectly with flavonols [21] . Using the same experimental model, Goncalves et al. [36] reported that antioxidant effects of the cherry samples were positively correlated with their levels of p-coumaroylquinic acid but negatively correlated with their cyanidin-3-rutinoside levels [36] .
The anti-glycation activity exerted by polyphenols has been investigated and a relationship structure-inhibitory activity of flavonoids has been demonstrated [7] . In particular phenolics acids and proanthocyanidins exert also anti-glycation effects triggered by MGO [34] [44] . A MGO-trapping effect of flavonoids has been also recently demonstrated [9] .
As far as concerns the physiological relevance of our results, previous studies have shown that cherry polyphenols are bioavailable [45] and their plasma concentrations range from 0.5 to 1.6 µM [46] . Moreover, previous studies have demonstrated that cherries exert antioxidant effects in vivo. Studies in human subjects, reported that the consumption of 280 g of cherries (about 45 sweet Bing cherries) increased plasma lipophilic antioxidant capacity [23] [24] . Moreover, in a double-blind, placebo-controlled crossover design, tart cherry juice intake (240 mL twice daily for 14 days) was associated with a decrease of F(2)-isoprostane levels, a marker of oxidative damage, in response to forearm ischemia-reperfusion. In the same subjects a decrease of urinary excretion of markers of oxidized nucleic acids (8-hydroxy-2'-deoxyguanosine, 8-hydroxyguanosine) was demonstrated [25] .
5. Conclusion
In conclusion, our results show that maturity stage modulates phenol content, antioxidant and anti-glycation properties of “visciola” extracts. Information about factors which modulate phenol content and the health-promoting components of “visciola” may help farmers to better promote their healthy properties and could lead to an increased consumption of these fruits, including their use as functional food.
Acknowledgements
The authors wish to thank AlfioSantinelli for the help in managing the experimental field and Maurizio Antinori for his effort in collecting and drying the fruits.
Abbreviations
NOTES
*Corresponding author.