Impact of Dye-Sensitized Solar Cell Anode Preparation on Performance ()
1. Introduction
The energy problem is the most restricting problem of current world economic development, solar energy as an inexhaustible, clean and pollution-free natural green energy, and becomes one of the most promising energies. Of currently solar cell research and application the most is silicon solar cell, but for the high cost of raw silicon cells, complex production process, improving efficiency potential is limited (the theoretical limit of the photoelectric conversion efficiency of 30%), limiting its civilian, in an urgent need of the development of low cost solar cell.
In 1991, O’Regan et al. [1] for the first time assembled photoelectric conversion efficiency of 7.1% - 7.9% of the DSSCs, and created a new field of solar cell research and development. Subsequently, Gratzel et al. [2] -[4] developed a photoelectric energy conversion efficiency up to 10% - 11% of DSSCs. As the DSSCs’ relatively low price, simple production process and potentially high photoelectric conversion efficiency, making it possible to replace the traditional silicon-based solar cells, it becomes the dominant future solar cells. The sensitized semiconductor optical anode of DSSCs plays a crucial role as a research hotspot. This paper introduced the preparation method of the anode of DSSCs, and the component parameters of two types of optical properties of anode effect are analyzed.
2. Experimental
2.1. Slurry Preparation of TiO2 Sol + P25
A certain amount of nano TiO2 powder is adding in TiO2 sol (nano TiO2 powder in volume in TiO2 sol was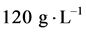 ), and add a certain amount of PEG (PEG an amount of 40% by mass of titanium oxide), sufficiently stirred, ultrasonic dispersion to obtain TiO2 slurry.
), and add a certain amount of PEG (PEG an amount of 40% by mass of titanium oxide), sufficiently stirred, ultrasonic dispersion to obtain TiO2 slurry.
2.2. Annealing
The coated membrane by placing the crucible in a muffle furnace at 20˚C/min at 500˚C heated to a constant temperature for two hours, then cooled in the furnace. After hydrolysis of the intermediate product suitable high-temperature annealing was completely decomposed, residual organic matter can be completely removed, and finally completely dehydrated, only closely integrated with the substrate of titanium dioxide films.
In order to study crystalline and optical properties of TiO2 thin films under different annealing process, the paper is in accordance with the annealing temperature was 400˚C, 450˚C, 500˚C, 550˚C experimental process of TiO2 annealing.
2.3. Pore-Forming Agent
In order to study the effects of pore-forming agent to TiO
2 film electrode spectral characteristics and optical properties, the molecular weight of 400 g/moL, 2000 g/moL, 4000g/moL PEG as the pore-forming agent to prepare TiO
2 sol-gel.
3. Results and Discussion
3.1. Observing the Morphology of TiO2 Films
With a scanning electron microscope (Hitachi Company, type S-570) to observe the surface morphology, Voltage of 20 kV, TiO2 ultrafine particles in the sol is TiO2 nanometer powder added P25 when preparing the slurry. Seen in Figure 1 (TiO2 film is a 500 times magnification scanning electron micrographs), the particles are very small, the specific surface area is large and surface roughness is high large, the film surface is uniformly smooth, particle agglomeration less. Interconnection between nanoparticles and the electrode constituting a sponge-like structure, so that there is good electrical contact between the nanocrystals, such injection of electrons from one particle to become another particle easier diafiltration. Between the spherical particles have numerous pores, and is conducive to the electronic transmission of the electrolyte; redox couple can penetrate into the porous film nanocrystal line semiconductor electrode, the dye molecules can be effectively oxidized regenerated.

Figure 1. SEM images of titania films (× 500).
3.2. Structural Testing of TiO2 Film
Using the RIGAKU D/MAX-2200 type PC X-ray diffraction of the phase of the sample were measured for the Cu target radiation. , the working voltage of 40 kV, current of 30 mA, the scanning range of 10˚ - 80˚, the scanning speed was 20˚C/min.
, the working voltage of 40 kV, current of 30 mA, the scanning range of 10˚ - 80˚, the scanning speed was 20˚C/min.
3.3. Effect of Annealing Temperature on Cell Performance
Figure 2 is X-ray diffraction pattern of TiO2 film sintered in 500˚C. Through calculation, the proportion of anatase and rutile from the before 82:18 becomes 77:23. Rutile content increased. Rutile TiO2 surface electron-hole pairs combine speed anatase faster than the relative anatase, its optical performance is poor. However, the band gap of rutile TiO2 is 3.0 eV, the band gap of anatase TiO2 is 3.2 eV, therefore, photosensitivity stronger than rutile TiO2 anatase. And found that due to the different crystal structures, can effectively promote the anatase crystals photoinduced charge separation of electrons and holes (mixed grain effect). Therefore, the appropriate content of rutile is advantageous. Scherrer formula calculation results show that the average grain size of anatase TiO2 nanoscale, which is about 18.9 nm, Compared to 18.6 nm before the sintering, only minor increases, grain growth is not obvious.
Figure 3 and Figure 4 are the effect of annealing temperature on the short-circuit current and open circuit voltage of the DSSCs respectively.

Figure 2. XRD patterns of titania films.

Figure 3. Relationship between the annealing temperature and the open circuit voltage.
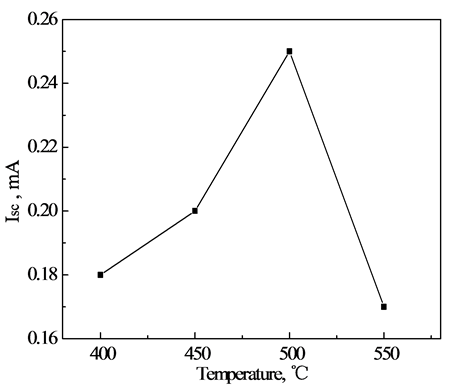
Figure 4. Relationship annealing temperature and short-circuit current.
As can be seen from the two figures, when the annealing temperature increased from 400˚C to 500˚C, the cell’s open-circuit voltage and short circuit current were increased, improved optical performance of the battery; When the annealing temperature reaches 500˚C, the short-circuit current and open circuit voltage reaches a maximum, respectively 0.60 mA and 0.6888 V; When the temperature continues to rise, the cell open circuit voltage and short circuit current is reduced. This shows that the annealing temperature on the optical properties of TiO2 thin film electrode is large, and there is an optimum annealing temperature. This is because the TiO2 film 400˚C annealed, the organic film is not completely removed, it will clog the holes in the TiO2 thin film, so the specific surface area decreased, the pore size decreases, reducing the dye adsorbed TiO2 film, reduces the cell short-circuit current.
Because the dye molecules is larger than the , the
, the  in direct contact with the TiO2 particles, the reaction tends to occur,
in direct contact with the TiO2 particles, the reaction tends to occur,  , reducing the open circuit voltage. However, when the annealing temperature is too high, the pore size increases. When the pore size is too large, the average distance between particles of TiO2 increases, the decrease in the efficiency of electron transport, electron injection flux decline, Shortcircuit current is reduced. From the test results, when the annealing temperature of 500˚C, the cell current and voltage reaches the highest value.
, reducing the open circuit voltage. However, when the annealing temperature is too high, the pore size increases. When the pore size is too large, the average distance between particles of TiO2 increases, the decrease in the efficiency of electron transport, electron injection flux decline, Shortcircuit current is reduced. From the test results, when the annealing temperature of 500˚C, the cell current and voltage reaches the highest value.
3.4. Effect of Pore-Forming Agent Molecular Weight on the Performance of the Cell
The TiO2 film electrodes prepared for optical performance testing, test results obtained are shown in Table1
As can be seen from the table, when adding a molecular weight of 4000 PEG, open circuit voltage and short circuit current density of TiO2 thin film electrodes were 0.5297 V and 0.35 mA/cm2. When adding a molecular weight of PEG 400, the open circuit voltage and short circuit current density of TiO2 thin film electrodes were 0.5864V and 0.49 mA/cm2, adding molecular weight is 2000PEG, open circuit voltage and short circuit current density was 0.6888 V and 0.60 mA/cm2, TiO2 film electrode has a better optical performance. This may be because when the molecular weight is 400 PEG decomposition annealing holes left too dense, thus the adsorption properties of the film do not substantially increase. When the molecular weight is 4000, PEG left decomposition annealing holes are too loose, in the annealing process and the film will be off, thus affecting the adsorption capacity of TiO2 film on dye. When the molecular weight is 2000, PEG annealing decomposition holes left loose moderate, TiO2 thin film on the adsorption capacity of the dye is better.
From the testing results, when adding a molecular weight of 2000 PEG, the cell has good photoelectric performance. The electrode assembly into the cell, photoelectric conversion efficiency test. When the incident light intensity is 73.1 mW/cm2, through  curves to calculate the maximum output power
curves to calculate the maximum output power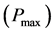 , using the formula:
, using the formula:


Calculate the photoelectric conversion efficiency  and fill factor
and fill factor  of 0.37% and 0.214% respectively.
of 0.37% and 0.214% respectively.
Figure 5 is a TiO2 thin film ultraviolet-visible absorption spectra, using PEG2000 as hole-forming agent. As can be seen from the figure, compared to pure TiO2 films, spectra of TiO2 films PEG2000 added in the shape of the curve after a changes, in the visible range of the light absorption has increased, expand the scope of the TiO2 nanoparticles absorb visible light direction. In addition, the absorption peak moves to long wave direction, A small amount ofred shift of spectrum, The light absorption properties increased.
3.5. Scanning Electron Microscope Observation
The SEM photos of the TiO2 film electrode is presented in Figure 6 and Figure 7 without PEG and join the PEG2000 prepared by sol-gel method, magnification 500 times. As can be seen from the figure, compared with the TiO2 film without PEG, after adding PEG2000, TiO2 nanoparticles and the surface of TiO2 film are bonded together to form a network structure, which is more conducive to electron transport, improve optical performance.

Figure 5. Ultraviolet-visible absorption spectra of TiO2 thin film with PEG2000 and pure TiO2 films.

Figure 6. SEM diagram of TiO2 films without PEG (×500 times).

Figure 7. SEM diagram of TiO2 films with PEG2000 (×500 times).

Table 1. Photoelectric properties of TiO2 films under different molecular weight PEG.
4. Conclusions
This paper focuses on the annealing temperature, pore-forming agent (PEG) molecular weight of the two parameters on the dye-sensitized TiO2 thin film photovoltaic solar cell performance, and with the X-ray diffraction, scanning electron microscopy, UV-visible spectrophotometer and other modern detection methods on the crystalline structure, surface morphology and optical properties of TiO2 thin films were characterized by the proceeds from the experimental results are as follows:
1) The effect of annealing temperature on the structure and optical properties of TiO2 thin films. Experimental results show that the annealing temperature of 500˚C, TiO2 film electrode has good optical properties;
2) By examining the different molecular weight PEG effect on the TiO2 thin film’s surface morphology, optical absorption properties, found that when adding PEG of molecular weight 2000, the TiO2 thin film electrode has the best performance. The experiment on the annealing temperature is 500˚C; process parameter added 0.5 g PEG, preparing the filling factor to be 0.39, the photoelectric conversion efficiency of dye-sensitized solar cells for 0.22%.
NOTES
*Corresponding author.