Do Epiphytes in Drier Climates Select Host Tree Substrates between Rough and Smooth Bole Textures and Crown and Stem, Vertical and Upright Stems? What Are the Implications for Water Catchment and Forest Management? ()
1. Introduction
Epiphytes require the support of physical structures for anchorage [1] . On trees, they occur on any part: trunk, branches and sometimes on the upper leaf surface (e.g. lichens), though Eleanor [2] recorded a higher preference for horizontal branches on which considerable depth of humus often collects. Kelly [3] and Cornellissen [4] argued against selection of certain positions and found that they rather depended on succession stage of the vegetation community implying that older forest communities held more epiphytes than secondary or lower seral stages. Yeaton and Gladstone [5] and Catling and others [6] supported the earlier assertion by Catling and Lefkovitch [7] that younger host trees which are characteristic of secondary forests supported fewer and different epiphytic associations usually non-vascular, from those supported by older substrates in older forest communities [1] . This was because vascular epiphytes required a deeper mantle of organic matter and a larger surface for anchorage [1] [8] and required a more humid environment characteristic of primary forests [1] .
All the above studies except for Chomba and others [1] were conducted in high rainfall tropical areas with high humidity approaching 100%. Therefore, this study further strengthens the earlier study by Chomba and others [1] which is now the second in roll to focus on epiphytes in Miombo woodland in Zambia, a vegetation community typical of the Zambezian Regional Centre of Endemism, which is poorer in tree species diversity, cover and humidity. It is here, in drier climates where the distribution of epiphytes is ecologically critical as it indicates rainfall regimes and their decline or disappearance would be an important ecological indicator of environmental disturbance or declining rainfall regime. In addition to being indicators of high rainfall and humidity levels, they also indicate primary/mature forest in which they play an important role of intercepting raindrops and aerosols such that in areas of increasing air pollution, they are the best aerial community from which the level of air pollution can be determined [1] . This study therefore, was carried out to further reinforce the earlier study [1] which did not cover in detail the influence of rough and smooth bole textures and stem inclination as important attributes characterizing distribution of epiphytes biomass in wet Miombo woodlands.
It was thought that results arising from this study would further reinforce earlier findings [1] and be used by Department of Forestry (FD), Zambia Wildlife Authority (ZAWA) and National Heritage Conservation Commission (NHCC), Zambia Environmental Monitoring Agency (ZEMA) and Local Communities to safeguard these important primary forests from excessive extractive uses, late fires and to guard against increasing pressure to have them developed for outdoor recreation, which may in the long term lead to loss of primary ecological services they provide. Specifically the research sought to determine the following: 1) epiphyte biomass pattern of distribution between rough and smooth bole substrates; 2) effect of trunk inclination on abundance and distribution of epiphytes; and 3) distribution between crown and stem.
2. Methods and Materials
2.1. Study Area
2.1.1. Location and Description
The Lusenga Plains National Park is located in Kawambwa District of Luapula Province. It is 880 km2 in extent and geographic location is longitudes 28˚55' East and 29˚12' South latitudes (Figure 1).
2.1.2. Climate
The Lusenga Plains National Park is located in one of the four high rainfall cells of Zambia receiving ≥1000 mm rainfall per annum and experiences; cool and dry season from April to August, hot and dry from September to October and hot and rainy from November to March/April. Mean annual temperature is 18˚C - 22˚C and

Figure 1. Location of study area, Lusenga Plains National Park and adjacent surroundings, Zambia (source: Zambia Wildlife Authority, GIS Laboratory).
monthly mean for June and July, which are the coolest months, is 15˚C - 17˚C and 23˚C - 28˚C for September and October which is also the hottest month of the year.
2.2. Field Methods
This study is part of the long-term monitoring programme established in the area before 2004. The study was carried out in Lusenga Plains National Park and adjoining waterfalls at Lumangwe, Kabwelume and Kundabwika waterfalls as part of the ongoing long-term study usually conducted every September 2004-2012/13. Woody vegetation types were sampled for epiphyte location/position on the host tree as earlier described and carried out [1] .
2.2.1. Biomass Distribution between Rough and Smooth Substrates
Epiphyte distribution between rough and smooth bole textures and stem inclination (Figure 2(a), Figure 2(b), Figure 2(c)) were collected while sampling along transects 1 km long which were located at random and laid parallel to each other to prevent crisscrossing. There was no clearing along transects as cutting of trees would be in direct conflict with the objectives of the study. Instead the researcher walked ahead of the team along an imaginary line with aid of a pair of campus (Figure 3).
 (a)
(a) (b)
(b) (c)
(c)
Figure 2. (a) Rough bole substrate of Pterocarpus angolensis with reticulately ridged scales like crocodile skin; (b) Longitudinal vertical fissures with raised oblong flakes of Terminalia spp.; and (c) Horizontal/inclined and upright trunks, yellow arrows represent upright stems and black arrows inclined trunks, Lusenga Plains National Park, Zambia.
Transect lines were separated by a minimum distance of 50 metres inside the National Park and 10 m near the waterfalls as in earlier study. Along each transect, quadrats 20 m × 20 m in size were stratified every 100 m and sampling was done based on the earlier approach [1] as modified after Gentry and Dobson [9] . Epiphytes found within the quadrat and their presence were allotted to rough or rough bole texture classes and stem inclination of upright and inclined (Figure 2(c)). Lichens, Usnea articulata and Ramalina reticulata which usually hanged on leaves to take advantage of rising mist from the water falls were left out as these due to their relative light weight, do not necessarily require big branches or stem for anchorage (Figure 4(a), Figure 4(b), Figure 4(c)).
The roughness or smoothness of the substrate was visually carried out to isolate these classes and further verified by running the inner side of the palm over the surface of the tree trunk. Rough bole texture had a prick scaly

Figure 3. Research team member armed with a rifle for protection in case of dangerous game takes compass bearing to mark transect, Lusenga Plains National Park.
 (a)
(a) (b)
(b) (c)
(c)
Figure 4. (a) Kundabwika Falls with mist rising which increases humidity and encourages growth of old man’s beard Usnea articulata and Ramalina reticulate; (b) and (c) Ramalina reticulata and Usnea articulata on tree crown taking advantage of the rising mist from falling water, Kalungwishi River.
feel (Figure 2(a), Figure 2(b)) on the palm and sometimes caused minor bruises or lacerations on the palm while the smooth bole did not. There were only two categories of rough and smooth bole textures developed and each substrate examined was subjectively allocated to either rough or smooth categories only. Stem inclinations was done visually as upright when the stem is near 180˚ and inclined when less than 180˚ including those lying on the ground. Stem inclination classification was practical as the researcher had earlier carried out a similar study on Mount Meru in Tanzania in 1993 and initiated the Lusenga Plains National Park epiphyte monitoring strategy. Epiphytes sampled along transects were recorded under the respective stem inclination and categories of rough and smooth bole textures.
2.2.2. Biomass
Tree canopies were accessed by climbing as earlier reported by Chomba and others [1] as modified after Nadkarni [10] and Tucker and Powell [11] . Epiphytes were removed from the host substrate using kitchen knife into carton boxes of various dimensions to determine volume. Wet weights were taken in the field by weighing each species with a solar weighing scale calibrated to the nearest gramme as in earlier study [1] . To get dry weight, epiphyte specimens were taken to base camp and dried to constant weight and reweighed to obtain dry weight, which was required in order to calculate biomass.
3. Results
3.1. Rough and Smooth Bole Textures of Substrates
Of all the tree species sampled, trees with rough boles texture had significantly higher biomass (dry weight) 1967 kg (89%) and smooth bole textured barks had the least 313.48 kg (11%) (Mann Whitney U test, P < 0.001) (Table 1, Figure 5), implying that rough barks provided better physical anchorage and therefore firmer support for epiphytes.

Table 1. Epiphyte host tree species selection and biomass between host species and vegetation communities.

Figure 5. Epiphyte biomass pattern of distribution between rough bole and smooth bole texture of substrate, Lusenga National Park, Zambia.
3.2. Epiphyte Abundance between Crown and Trunk
A total of 1805 kg epiphyte biomass was recorded. This biomass was partitioned between different host tree locations (Figure 6(a), Figure 6(b)). The distribution between host tree locations was significantly different being higher in crown and lower in trunk (Mann Whitney U Test, P < 0.05). Crown had 1220 kg (68%) and trunk 585 kg (22%) (Figure 6).
3.3. Epiphyte Distribution between Horizontal and Inclined Trunks
An evaluation of vertical and horizontal trunk/stem also showed that inclined trunks had significantly higher biomass 497 kg (85%) (χ2; P < 0.005) while upright stems/trunks had the least 88 kg (15%) and all the 88 kg were on rough bole textured trunks (Figure 7(a), Figure 7(b); Table 1).
4. Discussion
Epiphyte Biomass Distribution
Miombo woodland vegetation community is usually epiphyte deficient. They only occur in areas with rainfall ≥1000 mm/yr−1. This is because epiphytes are known to be very sensitive to humidity levels and are mostly found only in habitats where humidity is usually ≥80% [1] . This explains why they are concentrated around the three waterfalls, Lumangwe, Kabwelume and Kundabwika and in primary forest inside the National Park. At the water falls, they take advantage of the mist plume rising after the water plunges into the gorge below and these were mainly lichens. This humid condition maintains lower plants, in this case lichens and mosses.
In areas inside the National Park and away from the water falls, moisture and humidity are lower and this explains why epiphytes were more selective of host substrates. They only selected substrates that had capacity to enhance moisture retention. Crowns for instance had more dead organic matter which undoubtly was important to the survival of epiphytes as its water retention capacity provided a more continuous moisture supply for epiphytes than the atmosphere of bare or smooth bole [12] [13] . Inclined trunks on the other hand accumulated and retained humus and moisture than vertical branches. On upright stems litter would be easily washed away by rain water or blown off by wind currents, while the inclined stems and crowns had a further advantage of nitrates from the atmosphere [10] , mineralized dead organic matter accumulating on tree forks or invaginations on the stem and on inclined stems. This was in turn an important source of nitrogen for epiphytes and is probably a more reliable source than from the atmosphere [14] . It is for this reason that crowns and inclined stems which had branches, axils and invaginations in the host tree had the highest epiphyte biomass and supported epiphyte establishment (Figure 8). Although such trees may be considered to be of less timber value, their role in water retention and catchment functions is unsurpassed.
A vertical stem/trunk retains almost no wet season rainfall and experiences more rapid run off than an inclined or sloping trunk. On vertical trunks the influence of gravity is higher and less on sloping stems and this would directly or indirectly influence seedling settling and propagules.
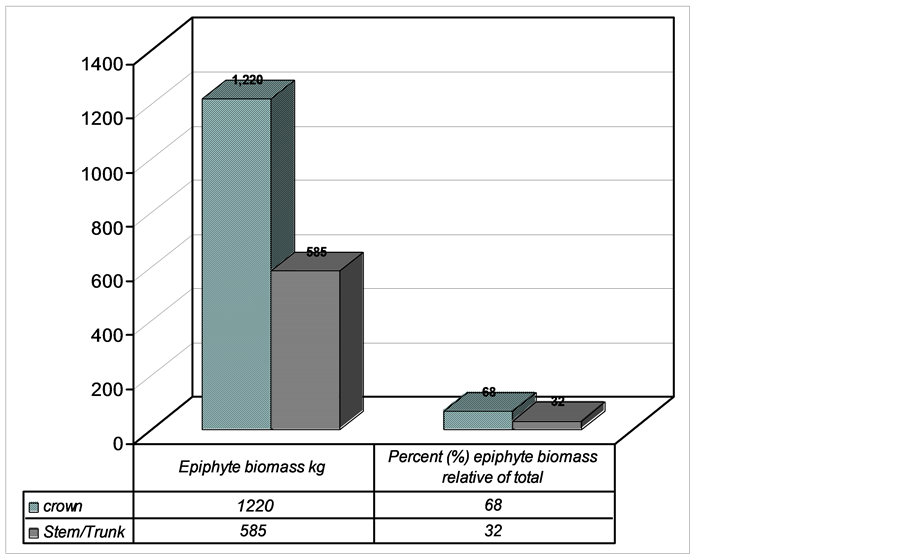
Figure 6. Epiphyte biomass pattern of distribution between crown (horizontal and trunk, Lusenga Plains National Park, Zambia).
On rough bole textured trunks, sometimes the bark peels off and if the trunk is inclined they may not fall off but settle and this combined with accumulation of moisture accelerates bark decay which provides exudates utilized by epiphytes. Barkman [15] noted that such conditions improved physical anchorage of seeds, spores, and propagules. Likewise, interception of light and water increased as the trunk becomes less and less vertical. Increasing sloppiness as already mentioned, promotes settling of seeds and spores and the accumulation of organic matter. Such conditions provided a suitable medium for epiphyte establishment [1] [15] and are critical water catchment attributes. These properties favoured horizontal trunks and rough bole textured substrates to have more epiphytes than smooth and vertical substrates.
A further explanation on the abundance of epiphytes in crown than trunk can be attributed to the following; crowns provide more micro-habitats than the trunk such as axils, forks, crevices, horizontal branches, and the cover of leaves also retains certain levels of humidity than bare upright trunks (see Figure 8). Earlier work by Gentry and Dobson [16] showed that epiphytes occurred on all trees in each stratum but were more frequent in the top storey, because epiphytes are light demanding. Trunks are generally poor in epiphytes except where trunks have several crevices on the stem or where the bole is convoluted as to allow for accumulation of litter, otherwise stems are usually impoverished in epiphytes because they lack the suitable properties for epiphyte establishment and are often shaded by the crown preventing light from reaching them [1] . Such findings are in agreement with the results of this study and emphasize the need for National Park authorities and the Forestry Department to manage primary forests in a manner that promotes increased crown cover and tree stem density.
5. Conclusions
It is concluded here that tree harvesting methods that target the crown would significantly alter epiphyte biomass and pattern of distribution. Such removal of tree crown would also impact on rain water retention capacity as epiphytes are known to be more efficient in intercepting rain water than ordinary foliage. Poc’s [17] and Chomba and others [1] indicated that epiphytes such as mosses were estimated to intercept rainfall at 400% - 500% of its dry weight in comparison to 60% - 175% of the dry weight for the foliage, which makes the presence of epiphyte in any catchment forest an important component of the ecosystem contributing to the biological stability of the landscape and ecological services.
 (a)
(a) (b)
(b)
Figure 7. (a) Epiphytes on horizontal/inclined stem with suitable anchorage providing stability and accumulation of litter which retains moisture for growth; (b) Epiphytes on upright/ vertical trunks were only on rough bole textured substrates and usually where there the trunk had branches or forked.

Figure 8. Host characteristics of forks, branches, invaginations, horizontal branching which are common in the crown support epiphyte establishment. Vitex doniana tree species with orchid epiphytes, Lusenga Plains National Park.
Trees should not be selectively managed on the basis of the stem structure and vertical alignments, which are usually thought to be of timber value, but for their ecological functioning. Trees that may appear to be of less timber value such as convoluted trunk, crevices in the trunk, sloping and many others should be retained for their ecological services such as water catchment.
Dead organic matter is undoubtly important to the survival of epiphytes as its water retention capacity provides a more continuous moisture supply for epiphytes than the atmosphere of bare bark. Decaying aerial organic matter is also converted into humus by fungi and micro-fauna contributing to fertility of forest soils. However, in areas with a long dry season (April to November) it can also be fuel for crown fires. Therefore, control of late fires may be absolutely necessary as these may promote crown fires which may subsequently consume aerial humus which forms a carpet on which epiphytes propagules and seedlings settle. Loss of such litter may make crowns drier as they would lose water retention capacity and become unsuitable for epiphytes. This may ultimately have a negative impact on the forests’ water catchment capacity.
Local communities in the surrounding areas should be sensitized through proactive awareness campaigns to carefully avoid fires escaping their fields as they prepare them for cultivation in the late dry season when ambient temperatures are highest. If such fires ran out of control and crossed into the National Park, they would annihilate aerial epiphytic communities. The use of fire in chitemene system of agriculture which involves pollarding, lopping and at times felling the whole tree stem should be discouraged in such areas, because it transforms primary forests to secondary forests, the latter of which is not suitable for epiphytes.
In view of the high levels of deforestation in Zambia currently being one of the highest in the Southern African sub-region together with Malawi [17] , ZAWA should consider extending the National Park boundary for Lusenga Plains National Park to include all the mature forests in the east of the Kalungwishi River in Mporokoso and Kaputa districts and all the riverine forests at the three waterfalls. This would secure and further enhance catchment functions of the area.
Acknowledgements
My colleagues particularly Professor Senzota of the Department of Zoology and Wildlife Conservation of the University of Dar es Salaam, Tanzania, provided guidance on epiphyte ecology. Park Ranger Mr. Peter Indala and all his staff worked and continue to work hard in monitoring vegetation in the National Park. They now take care not to destroy trees with epiphytes even when a new road has to be constructed they avoid cutting trees laden with epiphytes. I thank them for appreciating the role of epiphytes in water catchment functions and soil fertility in forest areas.