Thermodynamic Properties of Chromium (III) Ion Adsorption by Sweet Orange (Citrus sinensis) Peels ()
1. Introduction
The problems of the ecosystem are increasing with developing technology; heavy metals pollution is one of the main problems [1] . Toxic metal compounds coming to the earth’s surface can not only reach the earth’s waters (seas, lakes, ponds and reservoirs), but also contaminate underground water in trace amounts by leaking from the soil after rain and snow. Therefore, the earth’s waters may contain various toxic metals [1] . Heavy metals are non-biodegradable and can lead to accumulation in living organisms, causing various diseases and disorders [2] . It is well known that some metals are harmful to life, such as antimony, chromium, copper, lead, manganese, mercury, cadmium, etc., and are significantly toxic to human beings and the ecological environments [3] . Therefore, studies on the removal of heavy metal(s) pollution are increasingly challenging [1] . One of such methods is the use of adsorption. Interest has recently risen in the investigation of some unconventional methods and low cost materials for scavenging heavy metal ions from industrial waste waters [4] . In general, an adsorbent can be assumed as “low cost” if it requires little processing, is abundant in nature, or is a by-product or waste material from the industry [2] . Many researchers had worked on chromium and its effect on environment most especially when it is discharged through an industrial effluent. [5] observed the determination of chromium in tannery effluent. The adsorption of Cr (VI) on saw dust and charcoal from sugar cane bagasses by spectrophotometric method was conducted using diphenylcarbazide as a colour developing reagent to analyze the chromium content in tannery effluent located in Bara and Parsa districts of Nepal. According to [6] who carried out the removal of chromium in industrial effluent using ion exchange resins, effluent was discharged from the chromium electroplating industry containing a large number of metals, including chromium, copper, nickel and zinc; it was found that the ion exchange process is an alternative technique for application in the treatment of industrial waste water containing heavy metals. While [7] reported that the concentrations of Cu, Co, Cr, Cd, Mn, Zn, Ni and Pb in sediments of selected tanneries were measured. After digestion of the samples, the results of chemical analysis indicated that the concentration of most of these metals was higher than the values given by the national environmental quality standard levels. [8] conducted a systematic study of (silver/inorganic and/or organic halides) interphases based on cyclic voltammetric and impedance experiments carried out on polycrystalline electrodes of high reproductivity (controlled by comparison with monocrystalline ones), in water and in acetonitrile. The purpose of this study among others was to investigate the removal of chromium from aqueous solution by adsorption, to determine the optimum removal condition using orange peels (low-cost adsorbent), and to determine the suitable adsorption isotherms and their related thermodynamic and physical constants.
2. Materials and Methods
In the preparation of reagents, chemicals of analytical grade purity and distilled water were used. All apparatus were thoroughly washed with detergent solution followed by tap water and lastly, distilled water was used for the rinsing. Throughout the course of this work, distilled water was used significantly.
2.1. Sample Collection/Pretreatment
The adsorbent of interest in this work is powdered orange peels. The sample was collected from orange traders along Kabuga-Gwarzo Road, Kano State, Nigeria. The freshly collected samples were washed, air-dried, ground and screened to pass through a 1 mm analytical sieve to remove larger particles. The sieved sample was then labeled and put inside a polythene bag and stored in air-tight for further use as the adsorbent [9] .
2.2. Adsorption Envelopes Experiments
Adsorption experiments were carried out at different pH so as to ascertain the pH at which maximum adsorption was to be obtained. Adsorbent (2.5 g), Cr(NO3)3∙9H2O (1 g), NaNO3 (25 cm3, 0.1 M) differently at pH (2, 4, 6, 9 and 12) respectively were added into five different centrifuge tubes (50 cm3) with each tube conditioned at a different pH. The tubes were placed in a reciprocating shaker at 30˚C and shaken for four hours. The samples were centrifuged and filtered into labeled polythene bottles which were analyzed using the Atomic Adsorption Spectrophotometer (Buckmann Model 4) to determine the concentration of the residual (un-adsorbed) chromium [10] [11] .
2.3. Adsorption Isotherm Experiments
This experiment been temperature sensitive was conducted differently at 30˚C, 40˚C, 50˚C and 60˚C respectively. Adsorbent (1 g, 1.5 g and 2 g) were added differently into three different polypropylene centrifuge tubes (50 cm3). To each tube, NaNO3 (25 cm3, 0.10 M) at a pH of 2 (obtained as optimum pH from adsorption envelope) was added to obtain an ionic strength. Each solution was then allowed to equilibrate for a period of 24 hours (1 day). After equilibration, Cr(NO3)3∙9H2O (1 g, 0.10 M) was added to each tube and then shaken for a period of 4 hours differently at 30˚C, 40˚C, 50˚C and 60˚C respectively. The sample were then centrifuged at a relative centrifugal force of 8000 rpm (RCF = 7649 × g) for 20 minutes. The decantate was filtered and the solution was analyzed using Atomic Absorption Spectrophotometer (Buckmann Model 4) to determine the concentration of the residual (un-adsorbed) chromium. The same procedure was repeated for 0.20 M and 0.30 M chromium [12] .
3. Results and Discussion
The removal efficiency was determined by computing the percentage adsorption using the formulae in Equation (1)
 (1)
(1)
where, Ci = the concentration before adsorption and Cf = the concentration after adsorption [13] .
3.1. Effect of Initial pH (Adsorption Envelope)
In order to ascertain the pH at which the maximum adsorption of Cr (III) ions from aqueous solution occur, several experiments were carried out at pH of 2, 4, 7, 9, and 12 using 2.5 g powdered orange peel (as adsorbent) at a constant temperature of 30˚C. Table 1 presents the result as follows: it was observed that the adsorption process is dependent on pH. The percentage adsorption was found to increase with decrease in test solution absorbance value. It was also observed that increase in initial pH resulted to a decrease in percentage chromium adsorption onto the adsorbent with the optimal adsorption occurring at pH of 2. This could be associated to the reason that; of the three most common forms of chromium; Cr (II), Cr (III) and Cr (VI), the Cr (VI) is more common at low pH in form of  which is obtained by the oxidation of Cr (III) in aqueous solution. With the protonated groups present on the surface of the adsorbent at low pH, the negatively charged
which is obtained by the oxidation of Cr (III) in aqueous solution. With the protonated groups present on the surface of the adsorbent at low pH, the negatively charged  can easily be adsorbed by the positively protonated adsorbent surface, thereby increasing the adsorption tendencies of Cr (VI) to the adsorbent surface as pH drops [14] .
can easily be adsorbed by the positively protonated adsorbent surface, thereby increasing the adsorption tendencies of Cr (VI) to the adsorbent surface as pH drops [14] .
3.2. Adsorption Isotherm Models
Single-reaction models represent the simplest class of mathematical models describing solute retention/release reactions with adsorbent. These models can be broadly classified into kinetic and equilibrium types. The equilibrium models that was used in describing the adsorption isotherms for this research include; Linear, Freundlich, Langmuir and Temkin. Their mathematical expressions are as follows.
3.2.1. Linear Isotherm
 (2)
(2)
where S = adsorption density of chromium in the solid phase (μg∙g−1), Kd = distribution coefficient (mL∙g−1), C = concentration of chromium in solution (μg∙mL−1).

Table 1. Percent adsorption of chromium at various aqueous systems at varying pH.
3.2.2. Freundlich Isotherm
 (3)
(3)
where S = concentration of chromium retained by adsorbent, mg/kg or mol/kg, C = concentration of chromium in solution, mg/L or mol/L, K is a curve fitting parameter for equilibrium model, n = adsorption intensity [15] .
3.2.3. Langmuir Isotherm
 (4)
(4)
where S = concentration of chromium retained by adsorbent, mg/kg or mol/kg, C = concentration of chromium in solution, mg/L or mol/L, K is a curve fitting parameter for equilibrium model, b is a stoichiometric coefficient [15] .
3.2.4. Temkin Isotherm
 (5)
(5)
where C = concentration of chromium in solution at equilibrium (mg/L), X = amount of chromium adsorbed per unit weight of adsorbent (mg/gm), a and b are constants related to adsorption capacity and intensity of adsorption.
The adsorption experimental data were used to describe Linear, Freundlich, Langmuir, Temkin and DKR isotherm models. The goodness of fit of the experimental data was measured by the correlation coefficient, R2. At all the temperatures tested, Freundlich showed better fit followed by linear adsorption isotherm model as shown in Table2
If n is greater than unity, it indicates chemisorptions. Isotherms with n > 1 are classified as L-type isotherms reflecting a high affinity between adsorbate and adsorbent and indicative of chemisorptions [16] [17] .
In this work, the values of n are less than unity indicating physisorption. It can be concluded that since the experimental data best obey Freundlich isotherm and adsorption is physisorption, the adsorption is a typical multilayer and that the surface of the orange peel is heterogeneous [18] .
3.3. Thermodynamic Parameters and the Effect of Temperature on Adsorption
3.3.1. Arrhenius Equation
The effect of temperature on chromium ion adsorption by the orange peels can be examined in greater detail by applying the Arrhenius equation on the rate constant data. The mathematical expression of the Arrhenius equation is
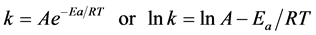 (6)
(6)
where k is the rate constant, A is the pre-exponential factor (frequency factor), which is a measure of the accessibility of the reaction sites to chromium, Ea is the Arrhenius activation energy, which must be overcome before adsorption can take place, R is the universal gas constant and T is the absolute temperature.

Table 2. Values of correlation coefficient (R2) for the adsorption isotherm models of chromium onto orange peels at various temperatures.
n = adsorption intensity in the Freundlich isotherm equation.
A plot of lnk (on the y-axis) and  (on the x-axis) yielded a straight line, from which A was obtained based on the intercept value [19] [20] .
(on the x-axis) yielded a straight line, from which A was obtained based on the intercept value [19] [20] .
3.3.2. Clausius-Clapeyron Equation
The determination of the isosteric heats of adsorption (ΔHr) as a function of adsorption density can also provide a clue to the uniformity or non-uniformity of adsorbent surface sites. The isosteric heats of adsorption were calculated from adsorption isotherms at 2 temperatures (30˚C and 40˚C) using the Clausius-Clapeyron equation thus:
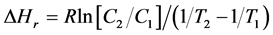 (7)
(7)
where ΔHr is the isosteric heat of adsorption at a given adsorption density, R is the gas constant, C2 is the equilibrium concentration of the chromiumions at temperature T2 at the given adsorption density, C1 is the equilibrium concentration of the chromiumions at temperature T1 at the same adsorption density.
If the isosteric heat of adsorption is independent of adsorption density, then the surface is homogenous. But if the isosteric heat of adsorption decreases with increasing adsorption density, then the surface is heterogeneous [21] . Also, if isosteric heat of adsorption is >40 kJ/mol, the process is chemisorptions and when less than 8 kJ/mol, it indicates the adsorption is physical in nature [22] .
The enthalpy change ΔH for adsorption was calculated using Van’t Hoff’s equation:

which upon integration yields:
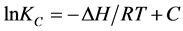 (8)
(8)
where C is a constant and ΔH does not change with temperature. Values of ΔH were computed from the slope of the linear variation of lnKC with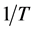 .
.
It has been observed that adsorption decreases with increasing temperature for the same mass of adsorbent and concentration. The decrease is as a result of the tendency of the chromium (III) ions to evaporate from the solid phase to the bulk phase or due to weak adsorptive forces between the active sites and the adsorbed species and also between close by molecules of adsorbed phase. This suggests that the adsorption process is physisorption. The isosteric heat of adsorption (∆Hr), is not independent of adsorption density and has all its values less than 8 kJ/mol, hence the surface was proposed to be heterogeneous. All values of enthalpy change were found to be negative indicating an exothermic adsorption process. The pre-exponential factor, A increases with increase in initial concentration of Cr (III) ions and adsorbent dose indicating that as the chromium (III) ion concentration and adsorbent dose increases, the greater the accessibility of adsorption sites to the Cr (III) ions (Table 3).

Table 3. Some thermodynamic parameters for the adsorption of chromium onto orange peels as adsorbent.
ΔH* and A*: average for the four temperatures.
3.4. Spontaneity of the Adsorption Process
Since
 (9)
(9)
where KC is the concentration equilibrium constant, Cs is the concentration of the chromium on the surface of adsorbent at equilibrium; Cb is the concentration of the chromium in the bulk solution at equilibrium. The entropy change ΔS of adsorption at equilibrium were calculated using the Arrhenius equation. The plot of lnKc as a function of 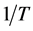 yields a straight line from which ΔH and ΔS were calculated from the slope and intercept, respectively. The results are given in Table 4 and Table5 The negative entropy change (ΔS) value obtained for most systems corresponds to less randomness at the solid/solution interface during the adsorption of chromium ions onto orange peels.
yields a straight line from which ΔH and ΔS were calculated from the slope and intercept, respectively. The results are given in Table 4 and Table5 The negative entropy change (ΔS) value obtained for most systems corresponds to less randomness at the solid/solution interface during the adsorption of chromium ions onto orange peels.
The thermodynamic parameter, Gibb’s free energy change ΔG is related to equilibrium constant KC by the equation:
 (10)
(10)

Table 4. Entropy (∆S/J∙mol−1∙K−1) values for the adsorption of chromium onto orange peels as adsorbent.

Table 5. Gibb’s free energy (∆G/J∙mol−1) for the adsorption of chromium onto orange peels.
where R is the gas constant, T is the absolute temperature; KC is the concentration equilibrium constant. This relationship has been found to be satisfactory for calculating the Gibb’s free energy changes involved in adsorption for low concentration systems. The change of free energy for physisorption is generally between −20 and 0 kJ∙mol−1, the physisorption together with chemisorption is at the range of −20 to −80 kJ∙mol−1 and chemisorptions is at a range of −80 to −400 kJ∙mol−1 [23] . As can be seen from Table 5, the overall standard free energy changes during the adsorption process were negative for the experimental range of temperatures, corresponding to a spontaneous and a physisorption process of chromium ions adsorption onto orange peels [23] .
4. Conclusions
This work described the sorption of Cr (III) ions from aqueous solution using sweet orange peels.
Based upon the experimental results of this study, the maximum removal efficiency of chromium (III) ions occurred at initial pH of 2 and solution temperature of 30˚C. Also, the removal efficiency of chromium (III) ions increases with increase in adsorbent dosage. The isotherm study indicated that Freundlich isotherm best modeled the adsorption process. The equilibrium removal of chromium (III) ions decreases as the temperature of solution increases. Thermodynamic studies revealed that the removal of chromium (III) ions by the orange peels is exothermic, feasible and the process is physisorption. Thus, orange peel is an effective adsorbent for the sorption of chromium (III) ions from aqueous solution.
NOTES
*Corresponding author.