1. Introduction
Among the main types of vegetation to be found in Mexico are pine forests (Ellis & Martínez-Bello, 2010). 56 species have been reported in the country (Farjon, 2001), placing it as having the largest number of pine species in the world. In the State of Veracruz alone, pine forests occupy an area of 57502.18 hectares at heights of between 1500 and 3000 m above sea level. These forests are dominated by species of the Pinus gender, although they may be associated with species of oak (Quercus spp.) (Ellis & Martínez-Bello, 2010).
Owing to their demand for timber, and agricultural activities introduced, the surface of temperate forest in México has been a reduction of between 127,000 and 167,000 ha/year, equivalent to approximately 0.5% to 0.8%/year, (Klooster & Masera, 2000), in addition to its being a source of raw material for industries employing terpentine, seeds, charcoal, pitch, pyroligneus acid, tar, methyl alcohol, among others (Martínez, 1992).
As an ecosystem, temperate coniferous forests, including pine forests, constitute a major asset in the mitigation of global warming due to the ecosystematic processes that they provide (Gerez-Fernández & Pineda-López, 2011), such as the protection of hydrological basins, soil retention, carbon capture, absorption of natural phenomena, climatic regulation and biodiversity protection (Zúñiga, Pineda-López, & Becerra, 2008). Nevertheless, human impact has had a great effect on these forests to such an extent that many of them have been converted into contemporary forests far less diverse and with less complicated structures (Jardel, 1991).
A conservational alternative for forest masses in Mexico has been the establishment of a National System of Natural Protected Areas (NPA) which is considered as an instrument for mitigating climate change (Beazury, 2009). For this reason, knowledge of the status of the forest mass, in terms of structure and composition, is ne- cessary for the definition of conservation strategies (Wadsworth, 2000; Urbieta, 2009).
The State of Veracruz has 80 Natural Protected Areas (NPA) which cover a total of 880935.8 hectares (Mo- rales-Mávil, Manson, & Márquez-Ramírez, 2001). One of these categories being the National Parks, of which there are 63 in Mexico, occupying a total area of 1,398,517 hectares, or 0.71% of the country (CONANP, 2013) and which include the National Park Cofre de Perote declared as such in 1937 (Diario Oficial de la Federación) in the most emblematic mountains of the State. This area has been considered as one of the 60 priority moun- tainous areas of the country (Zúñiga, Pineda-López, & Becerra, 2008). It is also included in the Forestry Resto- ration Program as a Priority Hydraulic Basin by the Secretaria de Medio Ambiente y Recursos Naturales (SEMARNAT) and represents an important Conservation Area for temperate forests which include one of their main species Pinus hartwegii Lindl.
Forests with species of P. hartwegii are to be found above 3500 m above sea level, a height which marks the altitudinal limit for these trees in Mexico (Miranda & Hernández, 1985). As is the case for other conifers, the population of this type of pine tree has been greatly reduced (Granados-Sánchez, López-Ríos, & Hernández- García, 2007) . At the present moment, it is under risk of extinction since, besides the human impact that it has had to withstand, it presents a low percentage of viability in its seeds (Iglesias, Tivo, & Casas, 2006) leading to a reduction of its reproduction rate making it vulnerable in the face of climatic change. According to Villers & Trejo (2004), a reduction of 49% is expected in its present distribution.
This species is one of the most widely studied species in the country (Musálem & Solis, 2002) as well as var- ious publications with a silvicultural focus, such as an estimation of the density of wood in the National Park La Malinche (Rojas & Villers, 2005); the determination of the external form of the trunk at the Ejido Galeana, Nueva León (Jiménez, Aguirre, Niembro, & Domínguez, 1994); an estimate of biomass and carbon in the aerial part (Jiménez, 2010) as well as adaptations in the face of fire of the species: morphological adaptations, survival or increase in height of saplings and resistance to prescribed burning (Rodríguez-Trejo, 2001; Castro, 2003; Ve- ra-Vilchis & Rodríguez-Trejo, 2007; Espinoza-Martínez, Rodríguez-Trejo, & Zamudio-Sánchez, 2008), in fo- rests in the State of Mexico in the National Parks of Iztaccihuatl and Popocatepetl.
Pinus hartwegii at lower levels, reaches a height of 20 m or more, but towards 4000 m the forest is more open and trees reach a height of 5 - 8 m (Rzedowski, 1983). At its maximum level, this species is affected by low temperatures (Musálem & Solis, 2000). The absorption of nutrients by the soil by the plants is slow which low- ers the rate of mineralization and nitrification of N (Marrs, Proctor, Heaney, & Mounford, 1988). The leaching of organic material in the soil is also increased (Tanner, Vitousek, & Cuevas, 1998) which in turn produces a re- duction in volume concentration of the majority of mineral nutrients. This, together with a reduction in habitat (Withmore, 1975) may lead to changes in the structure of the forest (Vázquez & Givnish, 1998).
The objective of this study was to characterize the structure according to size of a Pinus hartwegii forest along an altitudinal gradient (3500 - 4000 m above sea level) in the National Park Cofre de Perote. This species is to be found within this NPA at its altitudinal limit, so that an understanding of the status of its population will provide wider knowledge and a reference for future management.
2. Methods
2.1. Study Site
The Pinus hartwegii pine forest is to be found between 3500 and 4000 m above sea level (Vargas, 1997) within the National Park Cofre de Perote (PNCP), (19˚24'08" and 19˚32'04"N; 97˚05'07" and 97˚12'05"W) (INEGIORSTON, 1991). The climate is cold and sub-humid with a mean annual temperature of −2˚C to 5˚C and a mean annual rainfall of 1200 mm (Soto & Angulo, 1990).
2.2. Sampling
Following an altitudinal gradient, samples were taken from 3500 to 4000 m. above sea level and 20 quadrants 10 m × 10 m (two quadrants per 60 m in height), covering a total area of 2000 m2. These quadrants were sited along the north east slope which is more humid (Vargas, 1997). In each of these quadrants a geo-reference was taken at the central point with a GPS (Map 62Sc, Garmin, USA) in order to situate the UTM coordinates on a digital ortophotos scale 1:20,000, year 2004 (INEGI, 2004).
2.3. Evaluation of Variables
Each tree was numbered within each quadrant and its diameter was measured at breast height (DBH) using a 5 m tape (Forestry Suppliers, Germany) or at the base (DAB) in the case of trees under 1 m high. For trees with a fork, the following formula was applied:
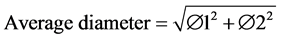 (1)
(1)
In order to measure the total tree height, an electronic clinometer (Haglof Sweden. 28616. Sweden) was used together with a flexometer. The canopy coverage was measured with a densiometer (Forest Densiometers, Model A) taking into account the area of the canopy and the crown coverage for each tree using a 30 m tape (Forestry Suppliers, Germany). The area was measured in s.q.m. taking the greatest and smallest diameter and applying the following formula:
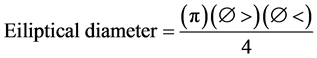 (2)
(2)
2.4. Statistical Analysis
A one-way ANOVA test was carried out in order to compare the density of tree individuals in relation to altitude using a normal distribution of data. In the case of the diameter, height and coverage differing from a normal distribution, a Kruskal-Wallis test (α = 0.05) was carried out. A Principal Components Analysis (PCA) was also performed with variations in diameter, height and crown coverage in order to determine which factors influence in distribution. All analyses were carried out using the statistical package STATISCA 7 for Windows.
3. Results
3.1. Forest Density
The ANOVA test did not establish any significant difference (p ≤ 0.05) between the treatments under evaluation (number of individual trees and height) F = 1.43. However, along the altitudinal gradient, the density of tree growth indicated three patterns: the first at an altitude of 3500 - 3600 m, where we encountered a tree density to a certain extent balanced, a second pattern at a height of 3656 - 3769 m, above sea level, and a third pattern at an altitude of 3824 - 3995 m, both showing a clear reduction in trees with increasing height. Some peaks can be appreciated with greater density at altitudes of 3550 m, 3656 m, and 3824 m, above sea level whilst at 3769 m fewer tree specimens were registered (Figure 1).
3.2. Diameter
Taking into account the diameter of the tree at breast height (DBH), no significant differences were encountered at the various altitudes (Kruskal-Wallis H = 11.02, p = 0.27). It was found that the majority of tree individuals were to be found in the first three categories, the most abundant examples were found to be 0.5 - 10.4 cm in di- ameter (49.4%) (Figure 2).
3.3. Height
In the case of the variable “height”, the Kruskal-Wallis test did not show any significant differences (H = 8.93,
![]()
Figure 1. Density of Pinus hartwegii in the Parque Nacional Cofre de Perote, Veracruz following an altitude gradient. The bars represent the mean ± SE.
![]()
Figure 2. Distribution by diameter size at breast height DAP (cm) of Pinus hartwegii along the altitudinal gradient. The bars represent the mean ± SE.
p = 0.44) between the altitude categories. However, it is graphically possible to identify that the majority of trees (97.7%) are to be found in the first four categories 0.2 to 12.4 m (Figure 3).
3.4. Canopy and Crown Coverage
The crown coverage did not show any significant differences in the Kruskal-Wallis test (H = 8.46, p = 0.48). Nevertheless, the majority of tree specimens were to be found in the first category of 0.01 sq.m to 15.2 sq.m— (88.9%) and are present along the whole of the transect at sites at 3656 m and 3684 m and can only be found in this category. In the higher categories they were scarce (only 11.04%) distributed in five categories (Figure 4).
![]()
Figure 3. The average distribution over the total height (m) of Pinus hartwegii, along the altitudinal gradient. The bars represent the mean ± SE.
![]()
Figure 4. The average distribution over the crown coverage (m2) of Pinus hartwe- gii, along the altitudinal gradient. The bars represent mean ± SE.
If we observe the categories in size per site, we find that, in general, at all sites there is a reduction in the number of trees as the diameter increases with the exception of those found at an altitude of 3600 and 3769 m above sea level. At the first of these sites, the second category (10.5 - 20.9 cm) was found to be the most abundant, whilst at the second it is the third category (21 - 31.4 cm) that is best represented (Figure 5).
We observe the distribution of height at each site, we find that this is heterogenic. The three sites at the lowest altitude (3500 - 3600 m) are represented by five of the six categories in size, although the proportion of each one of these varies since, with increasing height, there are less trees belonging to the first category (0.2 - 3.2 m), but these increase in the intermediate categories (6.3 - 12.4 m) (Figure 5). At altitudes of 3656 m, 2671 m, 3824 m, and 3920 m the majority of trees are seen to belong to the first category (0.2 - 3.2 m). At altitudes of 3684 m, 3769 m and 3995 m, the majority of individuals belong to the second and third categories. At the maximum altitude (3989 m), only trees of up to 9 m can be observed, whilst at lower altitudes, a greater diversity of sizes are to be seen. The tallest trees (18.6 m) were observed only at two sites (3684 and 3920 m).
The crown coverage found that larger sizes are only to 3556, 3824 and 3920 m in altitude, while lower category is present in all the sites (Figure 5).
The Principal Components Analysis (PCA) explains 58.4% of this variation according to two factors which are formed by the category of the size of the variables (diameter, height and crown coverage) and the number of individual trees (Figure 6). Three groups were formed: in the first, the altitudes 3500 m to 3684 m were to be found made up of sites where the greatest number of small specimens was to be found at the lowest level. The second 3716 m, 3769 m, 3920 m and 3995 m was where larger trees were observed at higher levels. A third group at the 3824 m site indicated a larger number of small trees at a higher level.
4. Discussion
Few large trees and many small trees were found in the Pinus hartwegii forest of the NPCP. This may be due to an historical explanation, since, during the 30 s, settlements were established on the mountain parallel to the
![]() (a)
(a)![]() (b)
(b)
Figure 5. (a) Distribution according to size (diameter in cm; height in m; crown coverage in sq.cm) per site range between 3500 m and 3684 m above sea level; (b) Continuation of distribution per site from 3716 m to 3995 m above sea level.
Decree covering the National Park. Since at this time, alternate periods of legal and illegal exploiting took place during the period of prohibition decreed from 1952 to 1978 and which continued to take place after 1982 until today (Vargas, 1997). This is probably the reason for the characteristic of this forest area as being the result of such exploiting. In this respect, Rojas (2004) mentioned that specimens of P. hartwegii with a diameter of more than 30 cm and a height of more than 15 m are probably more than 75 years old and can be considered as mature. In the same way, the presence of many small specimens assumes regeneration in the area, although it is not possible to foresee the survival of these without considering conservational measures for the area.
The number of individual trees per hectare at seven of the sites ranges from 2150 to 5300 per ha−1 which exceeds that encountered by Vera-Vilchis & Rodríguez-Trejo (2007) for the National Park of Cumbres del Aju- sco where the density of individual trees was found to be 900 to 2000 per hectare, considered there to be a high density. According to these figures, 50% of the sites studied by our group exceed the density reported for this species and suggests that these forests are under risk of fire.
In the case of the diameter at breast height (DBH), it was observed that the majority of individual trees (97%)
![]()
Figure 6. Graphical representation of the distribution of sites in accordance with the variables evaluated (diameter, height, crown coverage, density and altitude) use in the Principal Components Analysis.
are contained in the three lower categories. They are distributed in the form of an inverted “J” which helps in the regeneration of populations (Taylor & Halpern, 1991). This fact leads to the probability that populations are able to maintain themselves as part of a climax community (Barbour et al., 1987), always assuming that no other factors exist to modify this process, including fire. In the Sierra Nevada, the average diameter for P. Hartwegii is registered as 35 cm which corresponds to 44 years in age (Beaman, 1962). In the present study, the greatest diameter measured was found to be 61.5 cm; more than 42 cm in diamter specimens were scarce (17%), possibly due to the high density of individual trees/hectare, which suggests supression, where trees are not able to develop adequately. This condition could improve with the implementation of clearings in areas of greater density.
The majority of trees studied (70.4%) are smaller than 8 m high. According to Hernández, Rodríguez, Galle- gos, & Villavicencio, 2004, trees of this height are 8 to 9 years old. Only 29.6% exceed this height and no spe- cimen of 30 m was encountered, which is the maximum size that this species can reach according to Perry (1991). However, trees of up to 20 m have been reported in the NPCP, these being located on the eastern side where they are relatively less exposed (SEDARPA-UV, 2008).
García-Santiago (2005) refer to individual trees of up to 36.5 m with a diameter of 75 cm for the P. hartwegii forest at the Experimental Station of the Zoquiapan National Park on the Iztaccihuatl, in the State of Mexico. On the other hand, data that at the greatest altitude only specimens of 9 m exist as reported by Rzedowski (1983), who mentions that at 4000 m above sea level, this species present individuals of low rise as a result of environ- mental conditions, coincides with the results of the present study at an altitude of 3995 m where trees are no higher than 9.3 m with diameters of up to 41.9 cm.
It may be that at lower levels no tall trees have been found because these have been used for firewood (Iglesias, Tivo, & Casas, 2006; Vargas, 1997). Although firewood collection is not permitted within the Pro- tected Natural Area, nevertheless, there is evidence of this in the form of tree stumps or trees which have been used for the extraction of resin throughout the area. Pineda-López et al. (2013) who carried out an evaluation of this situation in the forest area known as Abies religiosa in the National Park, mention that the area has been subjected to the extraction of wood for domestic purposes as well as, to a lesser extent, for construction material. It is therefore possible that in the Pinus hartwegii forest something similar has taken place as it is situated next to the Abies.
In the analysis of the crown coverage, it was also observed that the majority of individual trees are small in size allowing for a greater amount of light to reach the ground stimulating the formation of a herbacious or bush undergrowth (Ávila-Bello, 2000; Granado-Sánchez, López-Ríos, & Hernández-García, 2007). In the case of our study site, the presence of Muhlenbergia macroura grass can be observed above 3656 m above sea level, whilst at lower levels (3560 to 3600 m) the canopy was found to be more closed (70% - 90%) and instead of grass, a covering of mulch was observed.
At altitudes of 3600 and 3789 m, few individual small trees were found regarding height, diameter at breast height and crown coverage, possibly due to some sort of disturbance which caused a temporary absence of re- generation as observed by Ajbilou, Marañon, & Arroyo (2003), in cork oak forests in the north of Morocco. In addition to these altitudes due to there is less moisture and greater influence of winds (Rzedowski & Rzedowski, 2005), limiting conditions for the growth of this species are created.
The low viability of P. hartwegii seeds (Iglesias, Tivo, & Casas, 2006) may be the cause of few seedlings, or an isolated distribution of these. Nonetheless may be due to predation, particularly in high areas with grassland with the abundance of rodents and rabbits considered as predetators of seeds and pine saplings influencing the stucture of the vegetation (Flores-Paredo, Sánchez Velázquez, Galindo-González, & Morales Mávil, 2011).
Fire is an additional factor which may contribute to the absence of seedlings, since, according to Rodríguez- Trejo (2001), this species of pine adapts to this factor. One of these instances being the regeneration of burnt sites, since fire favours the germination of seeds, but not so the establishment of germinated seedlings, and, with the elimination of grassland with which they could compete for space, the seeds are in contact with the soil and receive minerals from the ashes produced favouring germination. Franco & Sarukhán (1981), recognize fire as an important factor for the repopulation of P. hartwegii, in spite of the fact that saplings and plant seedlings of this species are susceptible to destruction by fire at this stage.
5. Conclusion
The characteristics of the forest show that it is heterogenious in its structure, there being few tall trees due to the exploitation of the area and most trees are small. Therfore Pinus hartwegii manifests a population structure in the form of an “inverted J”. However, although this may represent a greater quantity of young trees, in fact it is considered to be due to specimens that have been suppressed, since 50% of the sites indicate a high density and for this reason the forest is considered to be susceptible to fire.
Acknowledgements
Murrieta-Hernández wishes to thank the Consejo Nacional de Ciencia y Technologia (CONACyT) for a postgraduate grant No. 54427 awarded to Rogelio Lara González, Rafael Ortega Solis and Guillermo Vázquez for their cooperation in field work, as well as Jorge Morrales-Mávil for his patient revision and criticism of the do- cument.
NOTES
*Corresponding author.