1. Introduction
At the present time cancer still represents an enormous medical challenge. It is predicted that in this year in the USA about 580,350 persons will die from this disease. Worldwide breast cancer represents the main cause of deaths for women [1] and prostate cancer for men [2] . For this reason our attention is focused on finding a steroidal compound with the high anticarcinogenic activity [3] .
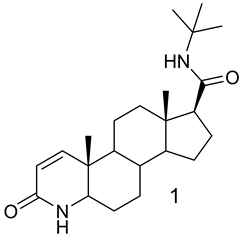
The most extensively studied class of 5α-reductase inhibitors is the 4-azasteroids, which include the drug finasteride 1. This compound is the first 5α-reductase inhibitor approved in the USA for the treatment of BPH. This compound has approximately a 100-fold greater affinity for type 2 5α-reductase, than for the type 1 enzyme [4] . In humans, finasteride decreases prostatic DHT levels by 70% - 90% and reduces the prostate size, while T tissue levels remain constant. The use of finasteride demonstrated a sustained improvement in the treatment of androgen-dependent diseases and it also reduced the prostate specific antigen (PSA) levels [5] .
In view of the fact that the 16,17-epoxy derivatives of 4,6-pregnadiene showed antiandrogenic activity [6] , it was of interest to synthesize the 3β-(p-iodobenzoyloxy)-16α,17α-epoxypregn-4-en-6,20-dione analog 7, and evaluate it as an antiandrogen [7] [8] .
In this paper we describe the synthesis and X-ray determination of 3β-(p-iodobenzoyloxy)-16α,17α-epoxy- pregn-4-en-6,20-dione 7. Compound 7 is prepared from the commercially available 16-dehydropregnenolone acetate and its synthesis is shown in Scheme 1. This compound has a high biological activity with a good probability to be used in the future for the treatment of androgen dependent diseases [9] [10] .
2. Experimental
2.1. Synthesis of the Compound 3β-(p-Iodobenzoyloxy)-16α,17α-Epoxypregn-4-En-6,20-Dione (7)
To a solution of steroid 6 (1 g, 1.74 mmol) in methylene chloride (8 mL) and pyridine (1 mL) was added dropwise under a nitrogen atmosphere at 0˚C thionyl chloride (0.2 mL). The resulting solution was stirred at room temperature for 45 min. Ice water (100 mL) was added and it was extracted three times with chloroform. The organic phase was washed with 10% aqueous hydrochloric acid, 5% aqueous sodium bicarbonate and water. It was dried over sodium sulfate and the solvent was removed in vacuum. The crude product was recrystallized from methanol. Yield of 87%. (KBr) cm−1: 3000, 2942, 1730, 1675, 1610. 1H-NMR (CDCl3) δ: 1.04 (3H, s, H-18), 1.3 (3H, s, H-19), 2.1 (3H, s, H-21), 2.7 (1H, H-16), 4.43 (2H, H-3), 6.6 (1H, H-4). 13C-NMR (CDCl3) δ: 18.7 (C-18), 20.1 (C-18), 67.6 (C-16), 69.9 (C-3), 79.5 (C-17), 129.1 (C-4), 147.8 (C-5), 201.6 (C-6). MS m/z 574 (M+).
2.2. In Vitro experiments
Cytotoxicity Assay
Compound 7 was screened in vitro against six human cancer cell lines: PC-3 (human prostate), MCF-7 (human breast), U251 (human glioblastoma), K562 (human erythromyeloblastoid leukemia), HCT-15 (human colorectal adenocarcinoma), SKLU-1 (humanlung adenocarcinoma). The cell lines were supplied by National Cancer Institute (USA). The human tumor cytotoxicity was determined using the protein-binding dye sulforhodamine B test (SRB) in microculture assay to measure cell growth, as described in the protocols established by the NCI. The cell lines were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 10,000 units/mL penicillin G sodium, 10,000 μg/mL, streptomycin sulfate, 25 μg/mL amphotericin B (Gibco) and 1% nonessential amino acids (Gibco) were added. They were maintained at 37˚C in humidified atmosphere with 5% CO2. The viability of the cells used in the experiments exceeds 95% as determined with trypan blue. The cells were removed from the tissue culture flask and diluted with fresh media. Of this cell
![]()
Scheme 1.Schematic representation of the formation of 3β-(p-iodobenzoyloxy)-16α,17α-epoxypregn-4-en-6,20-dione.
suspension, 100 μL containing 5000 or 10,000 cells per well, were pipetted into 96-well microtiter plates (Costar) and the material was incubated at 37˚C for 24 h in a 5% CO2 atmosphere. Subsequently, 100 μL of a solution of the test compounds obtained by diluting the stocks were added to each well. The cultures were exposed for 48 h to the drug at concentration of 50 μM. After the incubation period, cells were fixed to the plastic substratum by the addition of 50 μL of cold 50% aqueous trichloroacetic acid. The plates were incubated at 4˚C for 1 h washed with tap H2O, and air dried. The trichloroacetic acid fixed cells were stained by the addition of 0.4% SRB. Free SRB solution was removed by washing with 1% aqueous acetic acid. The plates were air dried, and the bound dye was solubilized by the addition of 10 mM of buffered Tris based (100 μL). The plates were placed on a shaker for 5 min, and the absorption was determinate at 515 nm using an ELISA plates reader (Bio-Tex Instruments) [11] .
2.3. In Vivo experiments
Animals and Tissues
Adult male golden hamsters (150 - 200 g) were obtained from the Metropolitan University in Xochimilco, Mexico. Gonadectomies were performed under pentobarbital anesthesia 30 days prior to the experiments and the animals were sacrificed with CO2. This protocol was approved by the Institutional Care and Use Committee of the Metropolitan University of Mexico (UAM). Human prostate from cadaver was kindly provided by Dr. Avissai Alcántara at The General Hospital (SS) in Mexico City, and stored at −70˚C. The prostate glands from hamsters were immediately removed, blotted and weighed prior to their use. Frozen human prostate was thawed on ice and minced with scissors. Unless specified, the following procedures were carried out at 4˚C. The animal and human tissues were homogenized with a tissue homogenize (model 985-370; variable speed 5000 - 30,000 rpm, Biospec Products, Inc.).
2.4. Determination of the Crystal Structure
X-ray data were collected on a Bruker Smart APEX AXS CCD area detector with a graphite monochromator and Mo Kα radiation (λ = 0.71073 Å) by the ω-scan method. The collected data were reduced using SAINT [10] and the empirical absorption corrections were performed using SADABS program [10] . The details were shown in Table 1.
3. Results and discussion
3.1. Refinement Details
All reflections were defined based on F2. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ (F2) is used only for calculating R-factors (gt) etc., and is not relevant to the choice of reflections for refinement. R- factors based on F2 are statistically about twice as large as those based on F, and R-factors based on all data will
![]()
Table 1. Data collection and handling.
be even larger. I1 and I1A atoms show some disorder and have occupancy factors of 0.55 (3) and 0.45 (3) and treated anisotropically.
3.2. Geometric Details
All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes.
3.3. Structure details
The structure was solved by direct methods [11] , and then refined by full-matrix least-squares technique; the position and anisotropic parameters of all the non-hydrogen atoms were obtained. The Flack [12] absolute structure parameter = −0.01 (2). The crystal structure is therefore determined with the correct absolute configuration and all relevant tables and figures are based on this configuration. Table 2 showed some crystal and refinement parameters. The crystal system of the title compound belongs to Monoclinic and the space group is P21, with cell parameters a = 10.8567 (11) Å, b = 7.5479 (7) Å, c = 16.0391 (16) Å, β = 109.473 (1)˚. Atomic coordinates and displacement parameters of the compound were shown in Table 3, from these data the structure was gained, which was shown in Figure 1.
The bond distances and bond angles are in good agreement with the corresponding values obtained in case of 16α-17-epoxy-4-pregnene-3, 20-dione [13] ; 21-hydroxy-11β,18-oxido-4-pregnene-3,20-dione [14] ; pregnenolone hemisuccinate [15] ; and 17α-acetoxy-6,7-epoxypregn-4-ene-3,20-dione [16] . Mean bond lengths for C(sp3)-C(sp3) and C(sp2) = O for compound 7 are 1.529 (7), 1.197 (7) Å, respectively.
In all essential details, the molecular geometry of 7 in terms of the bond lengths and angles are in good agreement with standard values. However, the C8-C9-C11, C12-C13-C17 and C14-C13-C18 bond angles of 113.5.1 (4), 118.7 (4) and 113.5 (4)˚ have been increased to minimize the steric interactions between the double bond at C4-C5 and the methyl groups at C10; and the interaction between the methyl group at C13 and the O atoms of the carbonyl oxygen atom at C17, respectively. The C20 carbonyl group eclipses C13-C17, the O3-C20-C17-C13 torsion angle is −2.8 (9)˚. The molecule is comprised of a quadricyclic skeleton consisting of three six-membered rings (A, B and C) and one five-membered ring (D). The molecule consists of three six-membered rings and one five-membered ring, all trans fused. According to the torsion angles and the puckering-parameters values (f2, θ2 and Q) [17] , the six-membered rings A (f2 = 3.21 (0.88)˚, θ2 = 48.29 (0.58)˚ and Q = 0.470 (0.005); Bθ2 = −154.70 (1.05)˚, θ2 = 27.01 (0.54)˚ and Q = 0.484 (0.004); and C f2 = −105.01 (3.57)˚, θ2 = 8.51 (0.50)˚ and Q = 0.573 (0.005) occur in an envelope (1E), deformed chair (1C4), and deformed chair (1C4)
![]()
Table 2. Crystal and refinement parameters.
conformations, respectively. Ring D occurs in a half chair conformation.
The absolute configurations of 7 for the chiral centers are 3S, 8S, 9S, 10R, 13S and 14S. The stereochemistry of the title compound is as follows: C8-βH is trans to C9-αH; C9-αH is trans to C10-βCH3; C13-βCH3 is trans to both C14-αH and C16-C17 epoxy ring.
Figure 2 shows the unit-cell packing arrangement for 7 along the b-axis. There are four intramolecular C-H・・・O interactions, which are due to their length, and could be considered as significant ones [18] [C12・・・O3, C18・・・O3, C11・・・O5 [−x + 1, +y + 1/2, −z + 1], C21・・・O4 [x + 1, +y, +z + 1], C25・・・O3 [−x, +y − 1/2, −z + 1] 3.100 (7), 3.324 (8), 3.126 (14), 3.324 (6), 3.211 (8) Å; H2B・・・O3, H18C・・・O3, H11A・・・O5, H21C・・・O4, H25・・・O3 2.589 (4), 2.811 (5), 2.232 (12), 2.544 (3) Å, 2.501 (5) and C12-H2B・・・O3, C18-H18C・・・O3, C11-H11A・・・O5, C21-H21C・・・O4, C25-H25・・・O3 113.04 (37), 114.38 (37), 152.73 (44), 138.38 (14), 133.10 (31)˚]. The molecules in the crystal are packed at normal van der Waals distances.
Table 4 shows the results for compound 7 in comparison to adriamycin which is used in chemotherapy. After castration, the weight of the male hamster prostates decreased (p < 0.005) as compared to that of the normal glands. Treatment with vehicle alone did not change this condition, whereas s.c. injections of 200 µg of testosterone for 6 days significantly increased (p < 0.005) the weight of the prostate glands in castrated male hamsters (Table 4). When testosterone and finasteride were injected together, the weight of the prostate decreased (p < 0.005) as compared to testosterone-treated animals (Table 5). However, when the same experiment was carried out with compound 7, the weight of the prostate significantly (p < 0.005) decreased.
The results from this study with six cancer cell lines and the weight of prostate’s hamsters showed very clearly that compounds 7 is a much better inhibitor for the enzyme 5α-reductase than the presently used Proscar 1 (finasteride).
![]()
Table 3. Atomic coordinates and displacement parameters (Å2).
![]()
Figure 2. The packing diagram for 7 projected along the b-axis.
![]()
Table 4. Effect of in vitro screening of compound 7 and adriamycin against six human cancer cell lines.
![]()
Table 5. Effect of in vivo of the synthetized steroid 7 and finasteride on the weight of prostate’s hamsters.
4. Conclusion
In this paper, the conformation of 3β-(p-iodobenzoyloxy)-16α,17α-epoxypregn-4-en-6,20-dione 7 was synthesized, and characterized by spectroscopic methods and its structure determined. X-ray diffraction analysis of (7) demonstrated that it consisted of four rings, three six-membered rings (A, B and C) and one five-membered ring (D). A, B, C and D rings occur in an envelope, deformed chair, deformed chair, and half chair conformations, respectively. The absolute configurations of 7 for the chiral centers are 3S, 8S, 9S, 10R, 13S and 14S. The crystal of 3β-(p-iodobenzoyloxy)-16α,17α-epoxypregn-4-en-6,20-dione is in monoclinic crystal system with space group P21, lattice constants: a = 10.8567 (11), b = 7.5479 (7), c = 16.0391 (16) Å, β = 109.473(1)˚, V = 1239.1 (2) Å3, Dx = 1.518 g/cm3 and Z = 2. The molecules in the crystal are stabilized by C-H・・・O interactions and van der Waals forces. Compound 7 has a high inhibitory activity for the enzyme 5α-reductase with a good probability to be used in the future for the treatment of androgen dependent diseases.
Supplementary Materials
CCDC-932617 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/cgi-bin/catreq.cgi, by e-mailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK, fax: +44(0)1223-336033.