1. Introduction
Along supercritical isotherms, the densities at which molecular clusters of occupied sites in the gas phase, or clusters of unoccupied voids in the liquid phase, first become macroscopic, are referred to percolation transitions. The respective limiting percolation transitions, denoted by PB and PA, refer to “bonded cluster” and “available volume” for gas and liquid phases respectively [2] -[4] . For real fluids, however, with continuous Hamiltonians, there is no unique molecular distance parameter to define a cluster, hence PA and PB are not generally defined at the molecular level. The percolation loci can, nonetheless, be defined thermodynamically along supercritical isotherms by the conditions


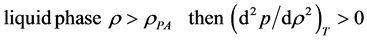
Thus, not only is there no continuity of liquid and gas, but the two equilibrium fluid states of matter are fundamentally different in their respective thermodynamic descriptions. Rigidity [(dp/dρ)T] is inversely proportional to fluctuations in the atomic number density. For a gas of attractive atoms, rigidity decreases with density because fluctuations increase with polymerisation, whereas for a liquid, rigidity increases with density because the voids become fewer as density increases. This condition also holds true for the subcritical isotherms of liquid and gas when T < Tc. To observe this intermediate mesophase that is neither pure liquid nor gas, we have investigated a model fluid of cohesive atomic spheres that gives rise to well-defined molecular clusters.
The energy of interaction between two square-well molecules i and j separated by distance rij is (see Figure 1)

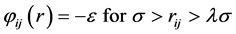

where σ is the hard-sphere diameter, ε is the square-well depth, and λσ is the range. The reduced thermodynamic states are then temperature  and density
and density

where kB is Boltzmann’s constant and N/V is the number density.
Interestingly, there is also an experimental manifestation of this model system in solutions of colloidal or protein molecules whose osmotic properties share a corresponding states law within McMillan-Mayer theory, with essentially the same phase diagram as molecular fluids [5] [6] . The adhesive model presented here is more relevant for charged colloids or proteins dissolved in aqueous solutions at moderate ionic strength. Under these conditions, the range of the pair potential is much smaller than the colloid diameter.
When ε à 0 the hard-sphere fluid is recovered. The percolation transitions of the hard-sphere fluid, as defined by Kratky [7] , have been computed [8] and found to be weak higher-order thermodynamic phase, transitions that occurs as the fluctuations in either number density, or equivalently available volume, change at the percolation

Figure 1. The square-well model atom-atom pair potential [φιj(rij)] with characteristic distances that define clusters, and hence percolation transitions, in the fluid phases: the available volume percolation transition PA associated with the distance 2σ is defined previously ([7] and [8] ); PB is the percolation transition associated with bonded clusters of atoms within the distance λσ.
threshold. The transition in the hard-sphere fluid (ε = 0) is so weak that, until recently it has been imperceptible from the equations-of-state. The effect of any added attraction of finite ε, however, is to strengthen the percolation transition with discontinuities in fluctuation-related second-order properties, heat capacity and compressibility, with decreasing temperature. Eventually, with sufficient cooling, at a “critical temperature”, the percolation loci intersect in the Gibbs pressure-temperature (p-T) plane. At this point, a liquid state at the density of the available-volume percolation transition coexists in thermodynamic equilibrium with a vapor state at lower density of a percolation transition associated with bonded clusters of gas molecules.
2. Molecular Dynamics
We have performed extensive molecular dynamics (MD) simulations, using a computer program DYNAMO, along several supercritical isotherms of an adhesive sphere fluid for λ = 1.005. A full report with details of these computations will be published elsewhere. DYNAMO is a modern event-driven MD simulations package developed by Bannerman [9] [10] and distributed under the General Public License. The full source code and documentation are freely available online at DYNAMO’s own web domains including dynamod.org, dynamod.co.uk and dynamod.com. DYNAMO sets out to calculate the smallest time to the next event. Within this time, the velocity trajectory of the events is then calculated by solving Newton’s equation of motion for the next event, e.g. collision of capture between any two atoms.
The results of the simulations are shown for the square-well fluid system of N = 10976 particles, i.e. (14 × 14 × 14 × 4) fixed initially on an FCC lattice, with periodic boundary conditions. The pressures along a supercritical isotherm T* = 0.215, shown in Figure 2, are obtained from independent runs of 70 state points along the isotherm in the region shown. Every state point is equilibrated independently for 10 million collisions and then simulated for 1 billion collisions with ensemble averaging of thermodynamic properties with averages taken over 1013 collisions where pressure is collected. In all, we have studied a total of 700 state point for 8 isotherms going from  until
until . By interpolating the slope in the mesophase, Tc can be determined and is found to be 0.200 ± 0.002.
. By interpolating the slope in the mesophase, Tc can be determined and is found to be 0.200 ± 0.002.
There is a connection between two coexisting phases at the critical temperature, and the percolation transition loci. The coexisting vapor density follows the loci that one expects, not for the hard-sphere excluded-volume percolation, but the extended-volume bonded cluster percolation transition of the square well. At the critical temperature and pressure, the vapor is at the bonded cluster percolation density ρPB, and the liquid at ρPA. At this critical temperature, both gas and liquid have the same chemical potential as the gradient in chemical potential (dp/ρ) approaches zero for densities between ρPB and ρPA i.e. as the density fluctuations diverge. At very narrow well-width approaching the adhesive-sphere limit (λ = 1.005), ρPE and ρPA are 0.30 and 0.45 respectively (Figure 2). This critical divide is narrower that for all other square-well fluids with wider attractive wells [2] .

Figure 2. Supercritical isotherm of the adhesive square-well model fluid (λ = 1.005) T/Tc = 1.075; the critical divide is shown as a dashed red line at the critical temperature Tc* = 0.200 ± 0.002; the straight line is added to clarify the mesophase linear region and the percolation transitions that bound gas and liquid phases.
MD configurations along the slightly supercritical isotherm (T* = 0.215) shown in Figure 2 have been carried out to determine the probability distribution of finding a particle in a cluster of size n at equilibrium. At very low density, i.e. for an ideal gas, there are no clusters and

where nc is the number of clusters. As the density increases, there are an ever-increasing number of pairs, then triplets etc., and increasingly large molecular clusters, until reaching a density when just one cluster of size N fills the whole box. At this point the probability function (nc − 1)/N goes to zero for large N. The cluster distribution for the intermediate density ρ* = 0.36, i.e. within the region of the mesophase, is shown in Figure 3.
The density-dependent % probability function P(n) can be defined by

where nc(n) is the number of clusters of size n. Multiplication by 100 is simply to avoid very small numbers on the log plots; the total probability normalizes to 100%. This is a normalized probability of a cluster of size n.
Figure 3 shows that there is clear mixture of gas phase and liquid phase in the supercritical mesophase. The gas component at the density 0.36 is characterized by a distribution of molecular sizes, monomers (12%) dimers (6%), trimers (4%), etc. with ever-decreasing probability, with an extremely small probability extending up to several hundred. The liquid phase, by contrast, shows just one cluster that is of the order of the size of the system, but variations in size in probability result as gas and liquid fractions fluctuate. The data in Figure 2 are indicative of a linear combination rule for thermodynamic state functions, which explains the linear equation-ofstate for the pressure p(ρ)T in the supercritical mesophase, as noted previously [2] -[4] , and seen here in Figure 2, for example.
Another more illuminating way to reveal the colloidal nature of the mesophase is by computer graphics, using color-coding for clusters of different sizes. In Figure 4, we show pictures of the molecular structures along a supercritical isotherm for densities corresponding to the gas phase (4a), the mesophase, on the gas side (4b) the mesophase on the liquid side (4c), and liquid phase (4d).
Figures 4(a) shows the gas molecules with a predominance of monomers (red) and all other sized molecules (brown); the largest molecules are around 30 atoms at this density, albeit with very low probability.
Figure 4(b) represents an equilibrium configuration within the mesophase at the density ρ* = 0.36. The colorcoding for the gas phase is the same as in Figure 4(a), except that the largest gas cluster, 267 atoms, is shown as yellow. There are two very large liquid clusters in this particular snapshot colored dark blue (4042 atoms) and

Figure 3. Distribution of clusters at the density ρ* = 0.36 for a supercritical isotherm at temperature T* = 0.215 (see Figure 2): the probability plot shows that all the atoms in the system belong either to a gas phase (molecules of less than 400) or liquid phase with clusters of the order of the system size >5000; the hiatus shows there is no intermediate cluster sizes, hence no “continuity between liquid and gas”.
second cluster colored light blue (1129). The important point to note is that every atom or molecule can be designated liquid or gas phase. There are no clusters of an intermediate size, i.e. there is no “continuity of gaseous and liquid phases”. We see a colloidal mixture that is nanoscopically heterogeneous but thermodynamically homogeneous, as distinct from the subcritical coexistence region that is thermodynamically heterogeneous.
In Figure 4(c), on the liquid side of the mesophase, ρ* = 0.4, the mixture of gas molecules and liquid cluster is also evident. The main difference between Figure 4(b) and Figure 4(c) is the gas side of the mesophase (4b), the dispersed phase is the liquid nanodroplets, (resembling a colloidal mist) whereas on the liquid side of the mesophase (4c) the dispersed phase is the nano-bubbles of gas (resembling a colloidal foam). Finally, for densities above the critical percolation threshold, as seen in Figure 4(d), there is just one large liquid cluster with a very dilute solution of gas like monomers or dimers.
3. Conclusions
In summary, recent discoveries of an alternative description of liquid-gas criticality have been extended for adhesive-sphere fluids. The revised phase diagram shows three equilibrium fluid phases. The “liquid” phase spans all temperatures from a metastable amorphous ground state to supercritical temperatures. There is a supercritical meso-phase bounded by two percolation transition loci and a gas phase. There is no “critical point” on the Gibbs density surface. When two percolation transitions have the same pressure, i.e. on intersection of the loci in the p-T plane, an equilibrium dividing line between the supercritical mesophase and subcritical coexistence is thermodynamically defined.
We have obtained revised results of the critical temperature (Tc) and critical pressure (pc) using only data from one-phase supercritical isotherms, and coexisting gas and liquid densities at Tc. Values of coexisting densities for adhesive sphere at Tc and the density loci of the percolation transitions that bound the existence of supercritical gas and liquid phases for T > Tc. These results confirm that there exists a supercritical mesophase as a fourth equilibrium state of matter after crystal, liquid and gas, with characteristic properties of a single-component colloid phase of gas in liquid or liquid in gas.
Finally, we note that a colloidal nature of the supercritical mesophase is consistent with the well-known phenomenon of critical opalescence. The present interpretation of the thermodynamics of criticality provides an alternative description of critical opalescence [11] observed in both molecular liquids and protein solutions [12] , known as Tyndall scattering, which is a manifestation of a colloidal nature arising from light scattering by colloidal particles with sizes comparable to the wavelengths of white light.
A detailed account of this research will be published elsewhere, and reported with more detail in the Ph.D. Thesis of HJM [13] .
Acknowledgements
We wish to thank the University of Manchester for the award of a Research Scholarship to HJM, and to acknowledge many helpful discussions with Drs. Marcus Bannerman and Leo Lue.