Remediation of Coastal Sediments by Addition of Calcium Nitrate and Presence of Benthos in a Muddy Tidal Flat ()
1. Introduction
Bioremediation is the process of a return to the original state that does not contain harmful compounds by using plants, fungi, microorganisms or their enzymes. Bioremediation has mainly been used to treat hydrocarbons, hexavalent chromium, aromatic hydrocarbons, diesel and crude oil in contaminated soils [1] -[5] . Despite many environmental remediation technologies being reported for land-based contamination, few studies have been conducted in aquatic environments. The removal of nitrogen from the aquatic environment will lead to the suppression of occurrences of red tide. Pore water exchange is lower in the muddy tidal flats compared with the sandy tidal flats to contain organic matter and fine particles of mud [6] . One of the important purification functions of tidal flats is nitrogen removal by nitrification and denitrification. At the tidal flat, the water depth is shallow, active biological motion and seawater exchange takes place, and enough ammonia and oxygen are supplied to nitrifying bacteria. Simultaneously, nitrate and organic matter are supplied to denitrifying bacteria [7] [8] . As a feature of the sediments of tidal flats, the environment is very different between the surface and the internal environment of the sediment. In the deeper part of the sediment, oxygen does not penetrate and an anaerobic environment is formed. In the environment, anaerobic, denitrifying bacteria are responsible for denitrification. In this study, we targeted the remediation of muddy tidal flat sediments that are generally an anoxic environment. Fine muddy sediments are under very strong reducing conditions and, permeability is small, but the environment can promote repair by creating different states. If mass transfer of oxygen and nitrate can be promoted, it is possible to reduce the acid-volatile sulfide and nitrogen by environmental remediation. Burrows of benthos contribute to oxygen transfer in tidal flats. The oxidation zone can be increased by penetration of fresh seawater to the nest holes of benthos. The sediment around a burrow will be oxidized but oxidation will be reduced with increased distance from the burrow. The denitrification process in this case is referred to as bioturbation [8] . Raymando et al. (1992) measured the denitrification activity in the bottom sediment using the acetylene block method, and reported the changes in relation to time and depth [9] . In this study, particle sizes are classified as fine muddy sediment rather than Raymando [9] . The method for enhancing the activity of denitrifying bacteria by supplying nitrate ions as well as changing the reduction state of mud flats with an oxidizing agent containing nitrates at the same time has been studied. Nitrate calcium hydrate is one of the oxidizing agents being used to improve sediments [10] . Addition of this compound to sediments could improve the oxidation of the sediment and accelerate denitrification activity [11] . We have also considered the effect of calcium nitrate on characteristics of muddy sediments in this study.
2. Materials and Methods
2.1. Effects of Bioturbation on Denitrification
To avoid the influence of original benthic organisms living in the sediment, they were excluded using a sieve. Introduced polychaetes were obtained from the same environment as the sediment samples as much as possible. Sediment samples were taken from the 0 - 2 cm layer. Sediment samples were processed through a 1 mm sieve by pouring seawater. Filtered Sediment was filled to a height of 20 cm into columns that had rubber stoppers on the bottom. Oxygen was slowly supplied into bulk seawater on sediments by an air pump. Figure 1 shows the experimental apparatus. The seawater was collected from the estuary tidal flat region (Figure 2). The sampling position was in Sagaprefecture Kyushu, Japan (130˚E and 33˚N). The seawater was replaced with newly filtered
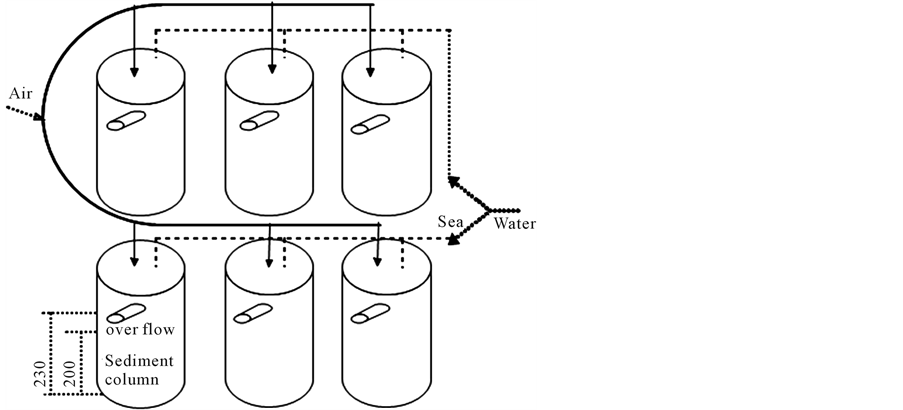
Figure 1. Experimental setup (RUN and Control).

Figure 2. Sampling point in Kyushu, Japan (130'24''E, 33'21''N).
seawater once a week. Six columns were prepared. Six polychaetes of 0.8 - 1.5 g were added to each of three columns. These columns were defined as RUN and the other three columns were defined as controls. Control and RUN columns were dismantled at days 15 and 30. Sediment COD and denitrification activity were analyzed.
2.2. Effects of Calcium Nitrate on Denitrification
In a separate experiment, calcium nitrate (5, 10 and 30 g) was added to each of three columns filled with sediment. A similar experiment procedure was conducted as in Figure 1, except that oxygen was not supplied in this experiment.
2.3. Analyses
Denitrification rate was measured by the acetylene block method [7] [10] [12] . Nitrous oxide was measured using a gas chromatograph (Hitachi GC-14, Tokyo Japan) with ECD detector. Redox potential of the sediment was measured using a platinum electrode (Eh portable meter PRN-41/Fujiwara Scientific Co. Ltd., Tokyo, Japan) with a diameter of 0.5 mm at a depth of 1 cm from the surface layer on the sampling time. Acid volatile sulfide was measured using the Sulfides Measuring Kit (Hydrotec-S, Gas Tech Corporation, Kanagawa Japan).
3. Results and Discussion
3.1. Effects of Bioturbation on Denitrification
Tables 1-3 show the results of denitrification rate, Eh and COD. Denitrification rate increased over time in the control. Redox potentials were −201 mV ± 40 mV in the control. Denitrification rate reached maximum values

Table 1 . Changes in the denitrification rate.

Table 2. Changes in the redox potential.

Table 3. Changes in the COD.
At 15 days, because of the large mass transfer and limited increase in oxidation state. The redox potential reached 120 mV at 30 days, and denitrification was reduced. Wang et al. [12] [13] reported that DO was inversely related to sediment denitrification rate.
Remediation of this sediment could be improved by using an oxidizing agent in the oxidation state from reduced state. Activities of benthos increased COD in sediment, but did not affect denitrification rate in this study.
3.2. Effects of Calcium Nitrate on Denitrification
Figure 3 shows the redox potential of the sediment column surface. Redox potentials were maintained at about −400 mV in columns with addition of 0 and 5 g calcium nitrate. In columns with 10 g and 30 g addition of calcium nitrate, the redox potential increased to −200 mV. Table 4 shows the nitrate nitrogen of interstitial water and denitrification rate of the surface layer. The denitrification activity was proportional to the nitrogen concentration [12] . Addition of calcium nitrate reduced sulfide content over the entire layer and strongly reducing conditions were improved. Yamamoto (2012) reported that crushed oyster shells contribute to oxidization of marine sediments, and acid volatile sulfide was reduced by addition of this powder [13] .
4. Conclusion
The muddy sediment in tidal flats has significant purification capacity, but the current situation is not optimal with low permeability and low redox potential. This study examined the environmental remediation of these conditions using biological and chemical treatments. We used polychaetes and chemical oxidation of calcium nitrate. Bioturbation altered the redox potential in muddy sediments to oxic conditions, and caused an increase in the denitrification rate. Redox potential increased with the amount of calcium nitrate added, but did not increase enough to reach an oxidized state. By addition of calcium nitrate to muddy tidal sediment, sediment COD

Table 4. Effects of calcium nitrate addition.
and acid volatile sulfide were also reduced, but because nitric acid remains in the bulk we will also consider the synergistic effects of bioturbation.