Adsorption Kinetics of Matter Contained in a Leachate Using Eggshell and Activated Carbon ()
The generation of highly polluting leachate from Sanitary Landfills has prompted the development of technologies applicable to treatment of these liquids. The aim of this study was to determine the kinetics of adsorption of organic and inorganic matter contained in pre-treated leachate (by advanced oxidation by the Fenton reaction); after two adsorbents were used, first eggshell and then activated carbon. To determine the content of organic and inorganic matter COD was measured; this was the parameter for monitoring the kinetics. The leachate was subjected to advanced oxidation treatment by the Fenton reaction, then the adsorption process was conducted by batch, in two consecutive steps, the first step was the use of eggshell and the second step activated carbon. Due to the oxidation treatment the pH value decreased to 2, after the adsorption eggshell the pH increased to 6.9 and this was maintained in the treatment with activated carbon. The kinetics of adsorption of organic and inorganic matter on eggshell was evaluated by models pseudo-first-or- der and pseudo-second-order, the better fitting was the latter. The adsorption process was adjusted to the model of Langmuir. The negative value of ΔHads indicated that the adsorption process was exothermic, spontaneous and favorable. The separation factor RL of Langmuir Isotherm values indicated that the adsorption of the matter on the eggshell was favorable at different temperatures. Finally, the activated carbon adsorption of liquid obtained from treatment with eggshell was favored with the kinetic model of pseudo second order. With the oxidation process, eggshell adsorption and activated carbon adsorption, the removal COD was 98.6%. The final COD value was fulfilled with the Mexican standard NOM-001-SEMARNAT-1996.
Keywords:
1. Introduction
The Sanitary Landfill is the most applied technology for solid waste disposing [1] . However the organic and inorganic substances contained in solid waste are released due to different chemical, physical and biological processes [2] , so that the generation of leachate (potentially polluting liquids) is the main disadvantage; more- over in the coming years increased production of solid waste and therefore a lot of leachate is expected, so that studies aimed at developing viable technologies to treat leachate are of paramount importance. Combination of physical and chemical processes is considered as the most appropriate treatment for the leachate with high concentrations of recalcitrant compounds [3] . The advanced oxidation process, especially using the Fenton reaction, is a treatment more applied for leachate [4] - [6] , as well as the adsorption processes, especially with activated carbon [7] . In order to reduce costs in the treatment of contaminated liquids, studies for reusing waste materials as bioadsorbents have been made, as the case of the eggshell [8] [9] . This waste is used because of its structural features, which include a 94% CaCO3, small amounts of MgCO3, Ca3(PO4)2 and a mucopolysaccharide protein [10] , in addition to this the eggshell is easily accessible worldwide. In adsorption processes it is very important to know the kinetics of adsorption in order to know the reaction time under the experimental conditions. Furthermore, the kinetic study is one of the most important parameter for the design and development of the adsorption to larger scale [11] . However to date there are not many researches that show the use of eggshell as adsorbent material for the matter contained in leachate; as well as studies and adsorption kinetics that govern this adsorption process on this adsorbent.
So the objective of this investigation is to determine the kinetics of adsorption of matter (organic and inorganic), contained in a leachate to be treated by an adsorption process with two adsorbents, first with eggshell and then with activated carbon. It is very important to mention that the leachate before applying the adsorption process received a pre-treatment by advanced oxidation by the Fenton reaction, following the methodology described by Barceló-Quintal et al. [4] .
2. Methodology
2.1. Sample Collection
Following the Mexican standard NMX-AA-003-1980 [12] , sampling was conducted in the lagoon landfill leachate in the municipality of Tlalnepantla, Estado de Mexico, Mexico.
2.2. Sample Analysis
The amount of organic and inorganic matter contained in the leachate was measured by chemical oxygen demand (COD), for which the samples were oxidized in the reactor HI839800 (HANNA Instruments, USA) based on Standard Methods 5520 C [13] ; subsequently a spectrophotometer DR/2400 (HACH, USA) was used, based on the Standard Methods 5520 D [14] . At the beginning and end of each stage the pH was determined with a computer interface Lab Quest (Vernier, USA), based on the Mexican standard NMX-AA-008-SCFI-2011 [15] .
2.3. Eggshell Characterization
The characterization of the eggshell was performed by spectroscopy, Fourier transform infrared (IR) on a Nicolet model iS10 infrared (Thermo Scientific, USA), by scanning electron microscopy (SEM) in electron microscopy sweep model JSM-6610 LV (JEOL, Ltd., USA) and X-ray spectroscopy (DXR) in X-ray equipment model D500 (Siemens, USA).
2.4. Leachate Oxidation
Untreated leachate samples were oxidized by Fenton’s reaction, according to the methodology described by Barceló-Quintal et al. [4] .
2.5. Adsorption Study on Eggshell and Activated Carbon
The batch adsorption process was performed in two consecutive steps the first was by using eggshell and the second with lignite activated carbon, as follow:
· For eggshell adsorption experiments were carried out by adding a fixed amount of this support (2 ± 0.3 g) in 40 mL of Fenton oxidized leached and shaken at 200 rpm in Orbital shaker, Lab-Line, USA, at temperature of 25˚C, for different contact times (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 16, 18, 20, 22, 24, 30, 48 and 72 h); Measuring COD at each time.
· The liquid from treatment with eggshell, was subsequently treated with activated carbon for its adsorption, the experiments were carried out by adding a fixed amount of support (2 ± 0.08 g) in 40 mL of liquid and shaken at 200 rpm on the Orbital shaker, to temperature of 25˚C, for different contact times (2, 4, 7, 10, 15, 20, 24, 32, 40, 48, 72 and 80 h); Measuring COD at each time.
2.6. Modeling of the Adsorption Kinetic
2.6.1. Pseudo-First-Order
The pseudo-first-order equation was used [16] :
 (1)
(1)
Integrating Equation (1) with respect to the boundary conditions from t = 0 until t = t and qt = qt the following equation is obtained [17] :
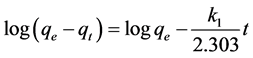 (2)
(2)
where qe and qt (mg∙g−1) are the amount of adsorbate material on equilibrium and in time t respectively, k1 (h−1) is the adsorption constant, plotted 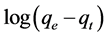 against t, resulting a linear trend for the kinetic of pseudo-first- order [17] .
against t, resulting a linear trend for the kinetic of pseudo-first- order [17] .
2.6.2. Pseudo-Second-Order
The pseudo-second-order equation was used [18] :
 (3)
(3)
Integrating the Equation (3) and applying the initial conditions the linearized equation was obtained:
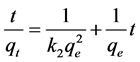 (4)
(4)
where k2 (h∙g∙mg−1) is the constant kinetic of the pseudo-second-order adsorption. By plotting t/qt against t, the value of qe is obtained through the slope and k2 by the intercept.
2.6.3. Intra-Particula Diffusion Model
For constant diffusion for intra-particular model, Kdif (mg∙g−1h−1/2), Equation (5) was used [19] :
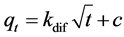 (5)
(5)
Plotted qt against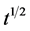 , c and kdif values can be obtained.
, c and kdif values can be obtained.
2.6.4. Study Adsorption Eggshell at Different Temperatures
The following eggshell weights were taken 3 ± 0.15, 4 ± 0.19, 0.20 ± 5, 6 ± 0.26, 0.23 ± 7, 8 ± 0.31, 0.44 ± 9 and 10 ± 0.51 g, with a particle size of 250 μm (+60, Tayler sieve type); 15 mL of leached previously oxidized was added, the contact time was 10 h, stirred at 200 rpm in Orbital shaker. The batch tests were performed by triplicate. Three different temperatures were tested 18˚C, 25˚C and 35˚C.
2.6.5. Langmuir Isotherm Model
Adsorptions were tested using the Langmuir isotherm model
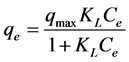 (6)
(6)
where qe (mg∙g−1) is the adsorbate concentration on the solid phase adsorbent in equilibrium, Ce (mg∙L−1) is the concentration of the adsorbate in the liquid phase at equilibrium, qmax (mg∙g−1) is the maximum amount of adsorbate per unit weight of adsorbent and KL (mg−1) is the Langmuir constant related to the affinity of the active sites.
The Equation (6) can be described as the linearized form [17] :
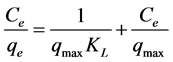 (7)
(7)
The constants were obtained by plotting  against
against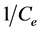 . Gouamid et al. [20] indicates; that essential feature of the Langmuir isotherm can be expressed in terms of the separation factor or balance parameter RL, which can be calculated from:
. Gouamid et al. [20] indicates; that essential feature of the Langmuir isotherm can be expressed in terms of the separation factor or balance parameter RL, which can be calculated from:
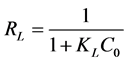 (8)
(8)
where C0 (mg∙L−1) and KL are the initial concentration and Langmuir constant, respectively.
The adsorption enthalpy calculation, DHads, at different temperatures was performed using the Van’t Hoff equation, which relates the variation of the absolute temperature (Kelvin grade) with the variation of the adsorption equilibrium constant (Kads) given by the difference in enthalpy (ΔHads) [21] :
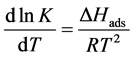 (9)
(9)
Assuming that the heat of reaction does not change with temperature, the resolution of this differential equation results in the following:
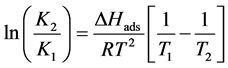 (10)
(10)
where R (J∙mol−1∙K−1) is the ideal gas constant (8.314 J∙mol−1∙K−1), T1 and T2 (K˚) are different temperatures, K1 and K2 are constants of adsorption at different temperatures. Considering the relationship between Gibbs free energy and the equilibrium constant (ΔG˚ = ΔH˚ − TΔS˚ y ΔG˚ = RTlnK, the equation could be written as follow:
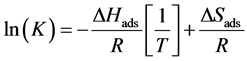 (11)
(11)
Thus, if is plotted ln(K) against![]() , it gives a straight line, where DHads can be obtained by the slope and DSads by the intercept.
, it gives a straight line, where DHads can be obtained by the slope and DSads by the intercept.
3. Results and Discussion
3.1. Oxidation Leaching
Prior to the adsorption treatment, the leachate was oxidized by Fenton’s reaction. To achieve good efficiencies of oxidation, it was necessary to consider four crucial steps: 1) Adjust the pH to between 2 and 4, 2) Optimization of the ratio of reactants![]() , 3) Coagulation-flocculation of sludge and 4) final pH neutralization [4] . The pH of the leachate was 8.53 ± 0.403 and was adjusted to a value of 3 by adding 0.008 mL of H2SO4 per 1 mL of leachate. The ratio
, 3) Coagulation-flocculation of sludge and 4) final pH neutralization [4] . The pH of the leachate was 8.53 ± 0.403 and was adjusted to a value of 3 by adding 0.008 mL of H2SO4 per 1 mL of leachate. The ratio ![]() was 0.125 [4] . The settled sludge was removed by filtration. The resulting pH after oxidation was 2 and whose neutralization was performed in conjunction with the adsorption process on eggshell, which showed the versatility that this residue has. The COD value of the untreated leachate was 7903 ± 2.58 mg∙L−1, after oxidation decreased to 1708.9 ± 93 mg∙L−1, with the latter value the adsorption process on eggshell began.
was 0.125 [4] . The settled sludge was removed by filtration. The resulting pH after oxidation was 2 and whose neutralization was performed in conjunction with the adsorption process on eggshell, which showed the versatility that this residue has. The COD value of the untreated leachate was 7903 ± 2.58 mg∙L−1, after oxidation decreased to 1708.9 ± 93 mg∙L−1, with the latter value the adsorption process on eggshell began.
3.2. Eggshell Characterization
The characterization of the eggshell was performed by infrared spectroscopy (IR), scanning electron microscopy (SEM) and X-ray diffraction (XRD). Figure 1 shows the IR of the eggshell. The characteristic peaks in the IR spectrum of eggshell found at 711.68, 871.35, 1394.62, 1794.86 and 3245.48 cm−1, which coincide with those reported by Witoon [22] . The peak at 1795 cm−1 indicates the presence of C = O (stretching), the 1397 cm−1 indicates, the presence of CO groups, both are characteristic of CaCO3.
Figure 2 shows the SEM of the eggshell, it can be seen a little porous structure attached at its membrane, this structure is responsible for the adsorption capacity of eggshell.
Figure 3 shows the XRD eggshell, it can see a crystal structure consisting mainly of CaCO3 calcite type.
3.3. Adsorption Kinetics of Leaching (Previously Oxidized) Using as Adsorbent Eggshell
The pH value was 2 to the start of the adsorption process and 6.9 at the end of such treatment, which shows that the use of egg shell has the ability to neutralize the pH of the oxidized leachate by Fenton, which prevents the use of any other reagent for this purpose, also the eggshell is a waste reusable, easy access, which its use allows to contribute to the resolution of solid waste management. In order to measure removal rates of organic and inorganic matter and the time at which equilibrium is reached, adsorption kinetics studies on eggshell as support were conducted.
Figure 4 shows the variation in the adsorption capacity at different contact times. The adsorption material was rapid in the first 10 h, the equilibrium being reached and it was maintained up to 14 h. When saturation of adsorbent was reached there was a stage where the remove is not presented. About 20 h, finally desorption process is presented.
With the experimental values for organic matter removing both models adsorption kinetics, pseudo-first-order and pseudo-second-order were applied to the results obtained in the first 10h, until the equilibrium time was reached. Figure 5(a) and Figure 5(b) show the linearity for pseudo-first-order and pseudo-second-order models, respectively.
With models of pseudo-first order and of pseudo-second order could not be made an accurate identification of the diffusion mechanism, so that the results were subjected to a kinetic analysis using the model of intraparticle diffusion, as shown in Figure 6.
Table 1 shows the results of the models used in the adsorption process with eggshell. The factor of linear correlation was higher for the pseudo-second-order model (0.9966), so that in the adsorption process with eggshell present best fit to this model. The chemisorption in this adsorption system is apparently present [23] . Moreover, the qe value obtained experimentally (17.98 mg∙g−1) with Langmuir isotherm was closest to that obtained applying the kinetic of pseudo-second-order (19.76 mg∙g−1).
On the other hand the linear correlation factor for the pseudo-first-order model (0.9007) is lower than that for the model of pseudo-second-order (0.9966), but its value is acceptable, indicating that the system could also be adjusted to this model, it could be explained due to the oxidation process of different compounds with different sizes which compete for the active sites of the support, some can be adjusted to a pseudo-first-order model and others can be adjusted to a pseudo-second-order model.
The linear correlation factor for intra-particle diffusion model (0.9167) was lower than for pseudo-first-order and pseudo-second-order models however its value is acceptable, so there are molecules in the system that can be adsorbed on eggshell following intra-particle diffusion kinetic.
The previous results indicated that the leachate is constituted by a mixture of molecules of different size and nature, and that each group of these molecules follows different adsorption mechanisms.
3.4. Equilibrium Adsorption Study
The adsorption study was performed with isotherm Langmuir; this model assumes that the adsorbate can form homogeneous monolayer on an adsorbent surface. This Isotherm was studied at three different temperatures 18˚C, 25˚C and 35˚C respectively, Figures 7(a)-(c).
Table 2 presents the constants obtained for the Langmuir isotherm evaluated at different temperatures.
![]() (a)
(a)![]() (b)
(b)
Figure 5. (a) Pseudo-first-order model and (b) Pseudo- second-order model.
![]()
Figure 6. Intra-particle diffusion model.
With the graph of Figure 8, DHads and DSads can be obtained. The value of DHads was −31.09 kJ∙mol−1, indicating that the adsorption is favorable [21] , also this value is greater than the heat released when there physisorption (less than 20 kJ∙mol−1) and less than the heat released when there chemisorption (100 to 500 kJ∙mol−1),
![]()
Table 1. Constants of the adsorption kinetics on eggshell.
![]()
Table 2. Langmuir isotherms at three temperatures.
![]()
![]() (a) (b)
(a) (b)![]() (c)
(c)
Figure 7. Linearization of Langmuir isotherm at different temperatures, (a) 18˚C, (b) 25˚C and (c) 35˚C.
showing that in this system there are two adsorption mechanisms, this result shows that there is a mixture of different components whit different sizes, resulting from the oxidation of leachate, so some of them are adsorbed by chemisorption and others by physisorption. The value of DSads is −167.47 kJ∙mol−1∙K−1, this value indicates that the adsorbed products are well ordered.
With the values of the Langmuir constants at the three temperatures the separation factors or balance parameter, RL, was calculated, Table 3. Gouamid, et al. [20] indicate that if RL = 0 the adsorption is irreversible, if 0 < RL < 1 the adsorption is favorable. Thus means that the adsorption of the adsorbates on the eggshell was favorable for all three temperatures.
The COD value decreased from 1708.9 ± 93 mg∙L−1 to 852.88 ± 35.82 mg∙L−1 with the eggshell treatment,
![]()
Figure 8. Linearization Van’t Hoff equation.
representing a 50% removal.
3.5. Adsorption Kinetics of Leaching (Adsorbent on Eggshell Previously) Using Activated Carbon as Adsorbent
In order to reach the Mexican standard NOM-001-SEMARNAT-1996 [24] , the activated carbon, lignite type, later was used as adsorbent, by their morphological characteristics, which also speeds removal of organic matter and studies adsorption kinetics were made of liquid that was obtained after the adsorption with eggshell. The final pH in the adsorption process on activated carbon was maintained at 6.9, indicating that the activated carbon has no effect on pH. Figure 9 variation shows in the capacity adsorption at different contact times. The adsorption of the materials was steadily increasing until reaching steady state at 48 h.
With the experimental values for the removal of different materials with activate coal until equilibration time (48 h), the adsorption kinetic models were applied the Figures 10(a)-(c) show the linearity for pseudo-first-order, pseudo-second-order and the intra-particle diffusion respectively.
Table 4 shows the results of the models used in the process of adsorption on activated carbon. Linear correlation factor for the model pseudo-second-order (0.9999) shows that the adsorption of the adsorbates on the activated carbon was adjusted to this model. The value of qe obtained experimentally (9.68 mg∙g−1) was very similar to that obtained by applying the model of pseudo-second-order (9.66 mg∙g−1).
As the results of adsorption on eggshell the linear correlation factor for the pseudo-first-order model was acceptable (0.937), indicating that the system could also fit this model. In addition, the linear correlation factor for the intra-particle diffusion model (0.9167) was good; so that in the system there are chemical species adsorbed on activated carbon, following the kinetics of intra-particle diffusion.
In the case of activated carbon adsorption also is observed that the trend was the meet the two pseudo-orders better one than another, this corroborate that leachate is formed by a mixture of chemical species of different size and nature, where these material follow different adsorption mechanisms.
With the oxidation process, eggshell adsorption and activated carbon adsorption, the removal COD was 98.6%, fulfilled with the Mexican standard NOM-001-SEMARNAT-1996 [24] .
4. Conclusions
In the oxidation process of the leachate it is crucial that the pH is adjusted to values between 2 and 4, and it is important that the relation ![]() in Fenton reaction must be optimized, sludge formed is separated and the resultant pH should be neutralized. The results show that the use of eggshell has the ability to neutralize the pH of the leachate oxidized by Fenton, which avoids the use of any other reagent for this purpose, it is also
in Fenton reaction must be optimized, sludge formed is separated and the resultant pH should be neutralized. The results show that the use of eggshell has the ability to neutralize the pH of the leachate oxidized by Fenton, which avoids the use of any other reagent for this purpose, it is also
![]()
Table 4. Kinetic constants of the activated carbon adsorption.
![]()
Figure 9. Adsorption capacity of activated carbon.
![]()
![]() (a) (b)
(a) (b)![]() (c)
(c)
Figure 10. (a) Pseudo-first-order model, (b) Pseudo-second-order model, (c) Intra-particle diffusion, All on the activated carbon.
reusable residue, easy access and its utilization can contribute to the resolution of solid waste disposal. Leachate is constituted by a mixture of chemical compounds with different characteristics and sizes. Two kinetic models were followed for the adsorption process on eggshell, one pseudo-first-order and the other pseudo-second-order; equilibrium data of the adsorption process were fitted better to the pseudo-second-order, by other hand, the kinetic was adjusted at the model of intra-particle diffusion. With the Langmuir isotherm the results of equilibrium adsorption functioned properly. The value of DHads indicated that the adsorption process was favorable. Its value indicates that in the system there are two mechanisms of adsorption, chemisorption and physicsorption, because in leachate exists a mixture of chemical species of different sizes and properties. The DSads value indicates that the products resulting on eggshell adsorption tend to be one order in the surface of this adsorbent. The liquid from adsorption in eggshell was adsorbed in lignite activated carbon, presented also the two kinetics, where the pseudo second order was best fit and the intra-particle diffusion model was also presented; which corroborates the presence of chemical species of different sizes and characteristics.
It is important and noteworthy that after treatment the value of organic and inorganic matter measured as COD meets the Mexican Standard NOM-001-SEMARNAT-1996.
Acknowledgements
Program Improvement Academic Staff (PROMEP) of the Secretariat of Public Education (SEP) by the support through the Network for Water Technology and the National Council for Science and Technology (CONACYT), which supports the PhD Graduates in Environmental Science and Engineering.