Reinvestigation of the Crystal Growth of “L-Proline Succinate” and “L-Threonine Zinc Acetate” Showing Use of Infrared Spectra for Product Identification ()
1. Introduction
Several groups are interested in the crystal growth and characterization of new non-linear optical (NLO) materials, which may be useful in several applications. α-Amino acids are ideal starting materials to react with several inorganic and organic acids and other reagents (for example metal salts) because 1) They contain reactive –NH2 or –COOH functional groups and 2) The chiral nature of the L-amino acids (excepting glycine) leads to the formation of compounds crystallizing in non-centrosymmetric space groups, which is a basic requirement for non-linear properties of compounds. Hence, several research groups venture into the synthesis of new NLO materials employing L-amino acids as starting materials. However, some basic facts on the reaction conditions and the analysis of the experimental observations are not taken into consideration, while publishing their results, leading to the publication of improperly characterized materials. This has been highlighted in several recent publications [1] -[5] . A comparison of the infrared spectra of the starting materials with that of the product formed is one useful method for proper characterization of a product crystal as will be demonstrated by taking two examples of published work on so called novel NLO crystals which are not novel but only unreacted starting materials.
In this work, it is convincingly proved that the X-ray data of the alleged L-proline succinate (1) reported by Paramasivam and Ramachandra Raja [6] in the paper titled, “Crystallization and Characterization of a New Non-Linear Optical Crystal: L-Proline Succinate (LPS)” and by Balamurugaraj, Suresh, Koteeswari and Mani [7] in the paper titled, “Growth, Optical, Mechanical, Dielectric and Photoconductivity Properties of L-Proline Succinate NLO Single Crystal” actually correspond to that of one of the parent chemical used, namely succinic acid. The crystals of a so called L-threonine zinc acetate (2) reported by Puhal Raj and Ramachandra Raja [8] in the paper titled, “Synthesis, Growth, Structural, Spectroscopic, Thermal and Optical Properties of NLO Single Crystal: L-Threonine Zinc Acetate” correspond to only the parent amino acid L-threonine.
2. Experimental Details
Commercially available L-proline (Himedia), succinic acid (Nice) and double distilled water were used for crystal growth from aqueous solution to obtain 1. Similarly, commercially procured L-threonine (Loba) and zinc acetate (Merck) were used for the crystal growth reaction to get the product 2. The IR spectra of the samples were recorded in KBr matrix in the frequency region of 400 to 4000 cm−1, using a Jasco spectrometer (FTIR, model 410) with a resolution of 4 cm−1.
2.1. Reinvestigation of the Synthesis of L-Proline Succinate
A mixture of L-proline (0.5756 g) and succinic acid (0.5904 g) was taken in the stochiometric ratio of 1:1 and dissolved in 10 ml of double distilled water and stirred well to obtain a clear solution. Then the solution was filtered and the clear filtrate left undisturbed for crystallization. Slow evaporation of solvent at ambient temperature (30˚C) resulted in the separation of transparent crystals after 15 - 20 days. The crystals thus obtained were labeled as compound 1.
2.2. Reinvestigation of the Synthesis of L-Threonine Zinc Acetate
L-Threonine (0.5956 g) and zinc acetate (1.0974 g) taken in the stoichiometric ratio of 1:1 were dissolved in 10 ml of double-distilled water to obtain a clear solution. The reaction mixture was filtered and kept undisturbed for slow evaporation to take place at ambient temperature (30˚C). After about 10 - 15 days, small crystals were formed, which were labeled as compound 2.
3. Results and Discussion
3.1. Usefulness of Infrared Method for Correct Product Characterization
The reported crystal growth of L-proline succinate 1 and L-threonine zinc acetate 2 is reinvestigated to unambiguously characterize the crystalline product obtained. The reinvestigation was undertaken due to the fact that the single crystal data reported by the authors were not accompanied by CIF file and the discussions presented indicated that the compounds are improperly characterized. In the case of compound 1 it was noted that the unit cell was identical to that of one of the parent materials used in the crystal growth reaction.
In present work, we have investigated the crystal growth reactions by employing the same conditions as in the earlier reported work and the product obtained is labeled as compound 1 (or 2). The products obtained in the crystal growth experiments were investigated by infrared (IR) spectra. As it is well documented that every compound exhibits a characteristic IR spectrum, a comparative study of the IR spectra of the starting reagents and the product material of a crystal growth reaction provides an useful method for product characterization. A new product crystal is expected to show differences in its infrared spectrum in terms of appearance of new signals or disappearance of existing IR bands in the spectrum of the starting reagents. The spectral changes will be pronounced if the molecular formula of a product differs considerably for example from succinic acid to L-proline succinate. The above reasoning has been made use of for accurate product characterization as described below.
3.2. L-Proline Succinate is Actually Succinic Acid
A comparison of the IR spectrum of 1 with that of the starting materials namely L-proline and succinic acid reveals that the spectrum of 1 is identical to that of pure succinic acid (Figure 1). The coincidence of the IR spectra clearly shows that no L-proline succinate crystal as claimed by the authors is formed in the crystal growth reaction and the product formed is succinic acid. Since succinic acid is a known compound and its spectrum is reported in the literature and IR spectroscopy is used as a characterization tool to infer new product formation, no discussion of the IR spectrum and band assignment is presented here.
Although the authors report to have grown by slow evaporation solution growth technique, single crystals of a so called nonlinear optical L-proline succinate from an aqueous solution containing L-proline: succinic acid in 1:1 mole ratio (Equation 1), based on the infrared evidence the crystal growth reaction can be correctly represented as follows:
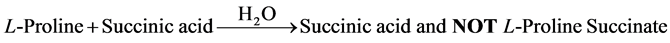 (1)
(1)
In accordance with the above infrared result, we note that the unit cell data reported by the authors of for their so called L-proline succinate crystal are in perfect agreement with those of succinic acid as shown in Table 1.

Figure 1. IR spectra reveal that succinic acid (black line) and the so called L-proline succinate (1) (blue line) are one and the same.

Table 1. Reported Single Crystal X-ray data on succinic acid.
Note – #No e.s.d. values reported; *No space group reported.
3.3. L-Threonine Zinc Acetate is Actually L-Threonine
A comparison of the IR spectrum of 2 with that of one of the starting material namely L-threonine reveals that the spectrum of 2 is identical to that of pure L-threonine (Figure 2). The coincidence of the IR spectra unambiguously shows that no L-threonine zinc acetate crystal as claimed by the authors of is formed in the crystal growth reaction (Equation 2).
 (2)
(2)
3.4. Fractional Crystallization
The formation of one of the starting materials as the product can be explained due to the process of fractional crystallization, when the reagents employed in a crystal growth reaction do not exhibit any chemical reactivity. The formation of succinic acid instead of 1 as product crystal in the crystal growth reaction can be rationalized based on the solubility data of the parent materials available in standard Handbooks, as 1 g of succinic acid dissolves in 13 ml of cold water . In contrast, L-proline is highly soluble with 162 g dissolving in 100 ml of water at 25˚C . Hence, from an aqueous solution containing both materials one can naturally expect the less soluble component namely succinic acid to crystallize first. The formation of succinic acid in near quantitative yield explains 1) No chemical reactivity between succinic acid and L-proline at room temperature 2) Our observation of identical IR spectra (Figure 1), and 3) the matching unit cell data reported by authors of with the reported data for succinic acid.
The formation of L-threonine crystal instead of 2 can also be similarly explained based on the solubility data of the parent materials. It is known that the solubilities of L-threonine and zinc acetate are 10.6 g and 31.1 g respectively per 100 ml of water at 20˚C . When there is no chemical reactivity between the reagents, it is normal to expect the less soluble component namely L-threonine to crystallize first, from an aqueous solution containing both materials.
Several instances of isolation and reporting of starting material or known compounds under the name novel NLO crystal are well documented in the literature in the form of critical comments - . Hence, we do not find the report on succinic acid crystals with the name L-proline succinate and L-threonine under the name L-threonine zinc acetate very surprising.
4. Conclusion
In summary, our above arguments show that the data given for the crystals formulated as L-proline succinate [6] -[7] actually concern succinic acid and hence one can rule out the formation of a new compound as claimed by the authors of [6] -[7] . Also, the data furnished by the authors on L-threonine zinc acetate [8] is on L-threonine.

Figure 2. IR spectra reveal that L-threonine (black line) and the so called L-threonine zinc acetate (2) (blue line) are one and the same.
The results described in this paper demonstrate the usefulness of IR spectral method for correctly identifying a product crystal.
Acknowledgments
SN thanks the Council of Scientific and Industrial Research, New Delhi for financial support under the Emeritus Scientist Scheme.
NOTES
*Corresponding author.